More Articles
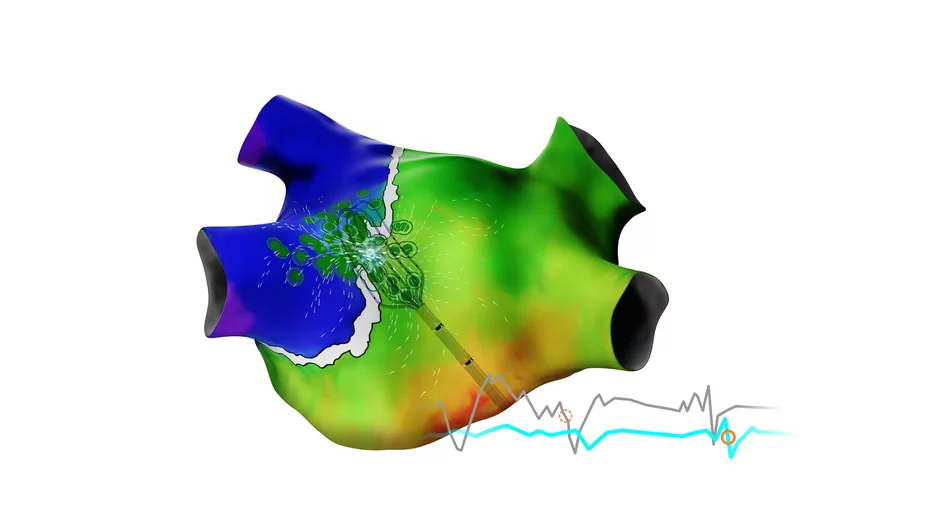
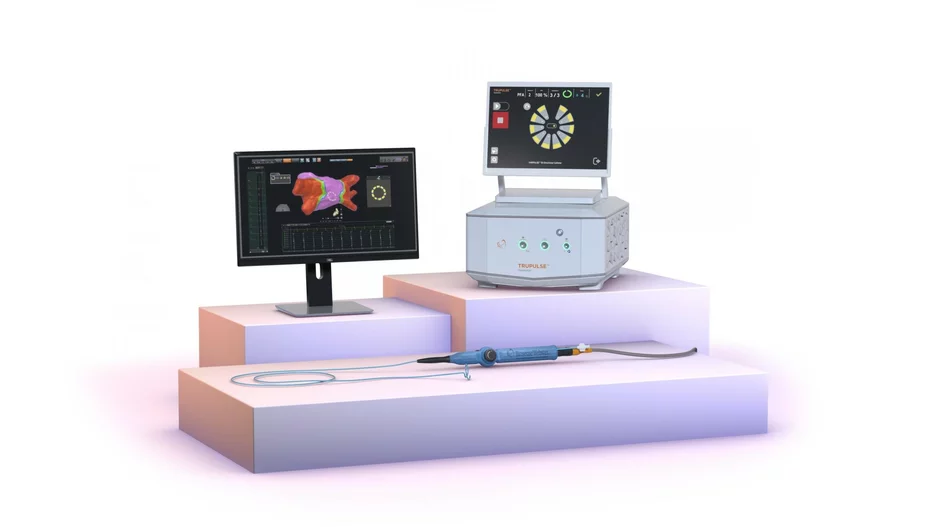
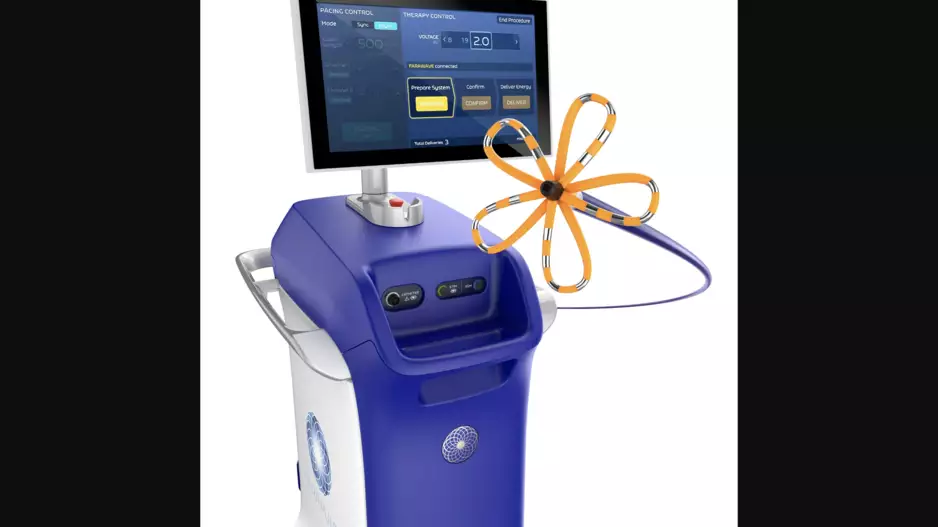
Biosense Webster, a part of Johnson & Johnson MedTech, has released the latest version of its Carto 3 3D mapping software for cardiac ablation procedures. The update, officially known as Carto 3 System Version 8, includes new modules designed…
Apple Watch AFib feature becomes first-ever digital tool approved by FDA to evaluate medical devices
The Apple Watch’s atrial fibrillation (AFib) history feature has qualified for the FDA’s Medical Device Development Tools (MDDT) program, meaning it can now…
Catheter ablation is known to benefit patients who present with atrial fibrillation (AFib) and heart failure with reduced ejection fraction (HFrEF). Its potential impact on patients with AFib and heart failure with preserved ejection fraction (…
Atrial fibrillation (AFib) is one of the most common heart complications in the United States, but many AFib patients fail to take the medications prescribed by their cardiologists. The American Heart Association…
Heart failure is the most common cardiovascular complication among patients with atrial fibrillation (AFib), according to new data published in The BMJ.[1] This may be a surprise to many, researchers said, because of how much attention…
Lexeo Therapeutics is one step closer to receiving approval from the U.S. Food and Drug Administration (FDA) for its new drug candidate for Friedreich's ataxia (FA) cardiomyopathy.
The New York City-…
Tecarfarin, a new anticoagulant from Cadrenal Therapeutics, has received the FDA’s orphan drug…
Biosense Webster, part of Johnson & Johnson MedTech, has submitted a premarket approval application for its Varipulse pulsed field ablation (PFA) system to…
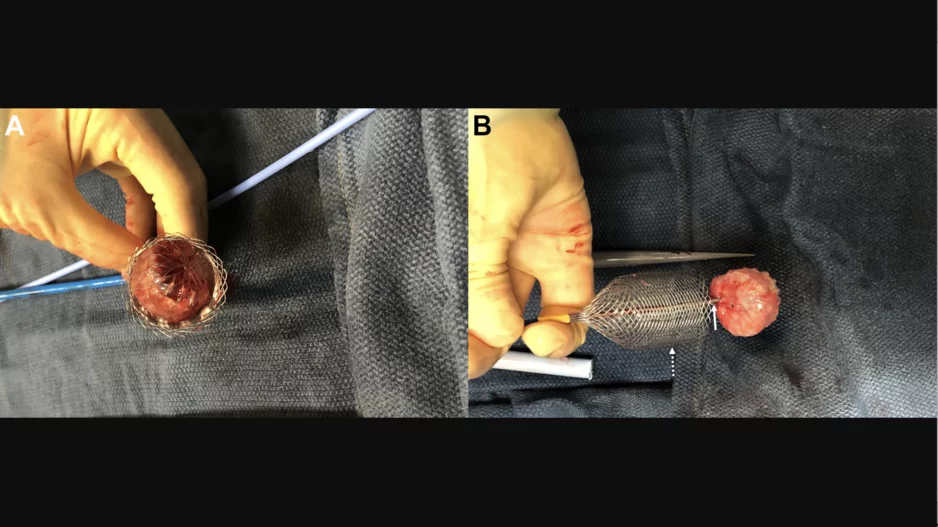
Interventional cardiologists in Houston used a new-look retrieval device to remove unstable left atrial appendage occlusion (LAAO) devices from three different elderly patients. The team wrote about its experience in JACC: Clinical…
Adults who regularly drink sweetened beverages face a heightened risk of atrial fibrillation (AFib), according to new data published in Circulation: Arrhythmia and Electrophysiology.[1]
The study’s authors reviewed data from more…
Implanting a permanent pacemaker (PPM) after transcatheter aortic valve replacement (TAVR) is costing health systems an average of more than $23,000…
Biosense Webster, part of Johnson & Johnson MedTech, has gained CE mark approval for its Varipulse pulsed field ablation (PFA) system, giving cardiologists…
Biosense Webster, a part of Johnson & Johnson MedTech, is funding two new collaborative studies focused on the use of its…
Scot Pollard, who played several seasons in the NBA and competed on “Survivor,” has been admitted to the ICU at Vanderbilt University Medical Center in desperate need of a heart transplant…
Continuous monitoring with Medtronic’s Reveal Linq insertable cardiac monitor (ICM) is a cost-effective treatment option for ischemic stroke patients with large artery and small vessel disease, according to new data being presented at the 2024…
Biosense Webster, part of Johnson & Johnson MedTech, shared updated data on its Varipulse pulsed field ablation (PFA) system at AF Symposium 2024 in Boston…
Targeting the left atrial posterior wall (LAPW) when patients with persistent atrial fibrillation (AFib) undergo pulsed field ablation (PFA) does not significantly improve outcomes, according to new research presented at AF Symposium 2024 in…
Medtronic announced Thursday, Feb. 1, that commercial patients around the world are now being treated with its PulseSelect pulsed field ablation (PFA) system.…
Boston Scientific’s Farapulse Pulsed Field Ablation (PFA) System has been approved by the U.S. Food and Drug Administration (FDA) to treat patients with symptomatic, paroxysmal atrial fibrillation (AFib), making…
A new-look medical device may reduce the risk of injuries to the esophagus during radiofrequency ablation (RFA) procedures for atrial fibrillation (AFib), according to new research published in JACC: Clinical Electrophysiology.[1]
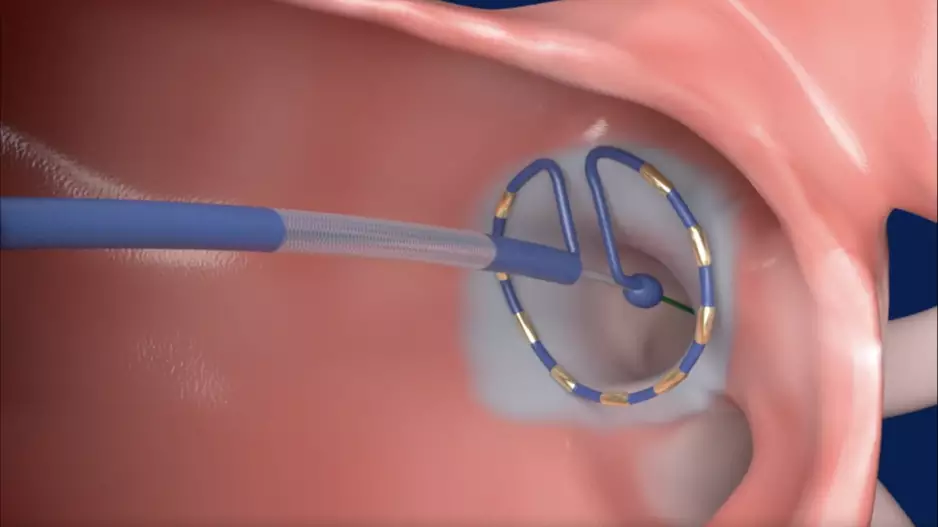
…Element Science, a San Francisco-based medical device company, has gained CE mark approval for its new patch-based cardioverter defibrillator.