More Articles
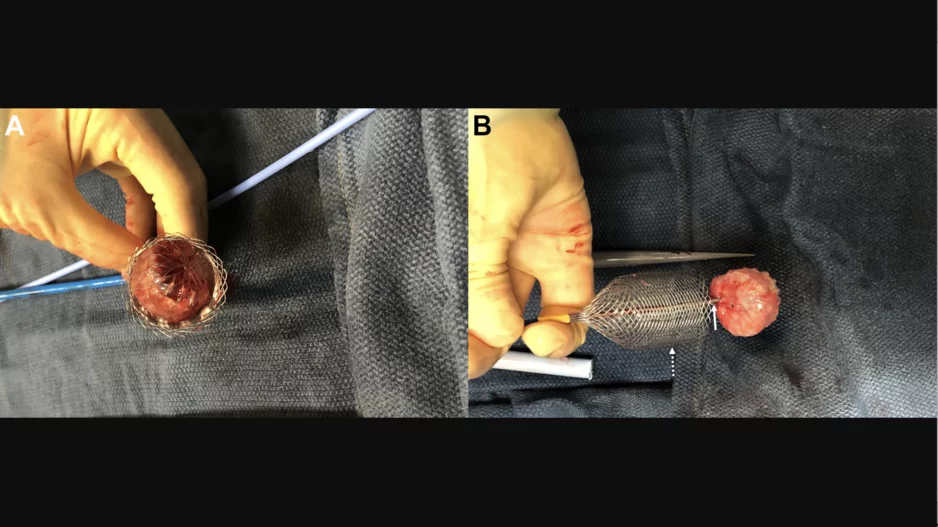
Interventional cardiologists in Houston used a new-look retrieval device to remove unstable left atrial appendage occlusion (LAAO) devices from three different elderly patients. The team wrote about its experience in JACC: Clinical…
Adults who regularly drink sweetened beverages face a heightened risk of atrial fibrillation (AFib), according to new data published in Circulation: Arrhythmia and Electrophysiology.[1]
The study’s authors reviewed data from more…
Implanting a permanent pacemaker (PPM) after transcatheter aortic valve replacement (TAVR) is costing health systems an average of more than $23,000…
Biosense Webster, part of Johnson & Johnson MedTech, has gained CE mark approval for its Varipulse pulsed field ablation (PFA) system, giving cardiologists…
Biosense Webster, a part of Johnson & Johnson MedTech, is funding two new collaborative studies focused on the use of its…
Scot Pollard, who played several seasons in the NBA and competed on “Survivor,” has been admitted to the ICU at Vanderbilt University Medical Center in desperate need of a heart transplant…
Continuous monitoring with Medtronic’s Reveal Linq insertable cardiac monitor (ICM) is a cost-effective treatment option for ischemic stroke patients with large artery and small vessel disease, according to new data being presented at the 2024…
Biosense Webster, part of Johnson & Johnson MedTech, shared updated data on its Varipulse pulsed field ablation (PFA) system at AF Symposium 2024 in Boston…
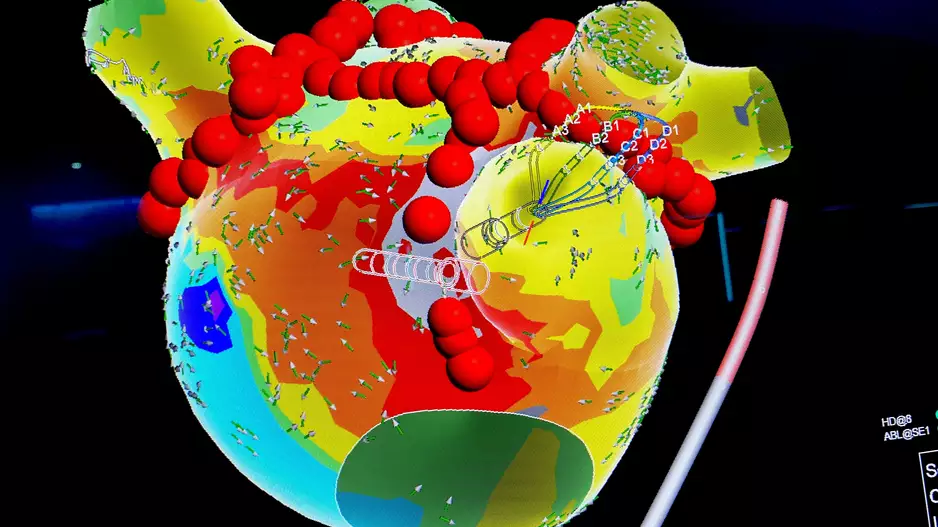
Targeting the left atrial posterior wall (LAPW) when patients with persistent atrial fibrillation (AFib) undergo pulsed field ablation (PFA) does not significantly improve outcomes, according to new research presented at AF Symposium 2024 in…
Medtronic announced Thursday, Feb. 1, that commercial patients around the world are now being treated with its PulseSelect pulsed field ablation (PFA) system.…
Boston Scientific’s Farapulse Pulsed Field Ablation (PFA) System has been approved by the U.S. Food and Drug Administration (FDA) to treat patients with symptomatic, paroxysmal atrial fibrillation (AFib), making…
A new-look medical device may reduce the risk of injuries to the esophagus during radiofrequency ablation (RFA) procedures for atrial fibrillation (AFib), according to new research published in JACC: Clinical Electrophysiology.[1]
…Element Science, a San Francisco-based medical device company, has gained CE mark approval for its new patch-based cardioverter defibrillator.
Performing left atrial appendage occlusion (LAAO) at the same time as other cardiovascular interventions does not increase the patient’s risk of major adverse events, according to new research published in JACC: Cardiovascular Interventions.[1]…
Abbott announced this week that more than 30 patients in Australia were treated with the company’s new pulsed field ablation (PFA) system, representing the first time the technology has been used to treat arrhythmias such as atrial fibrillation…
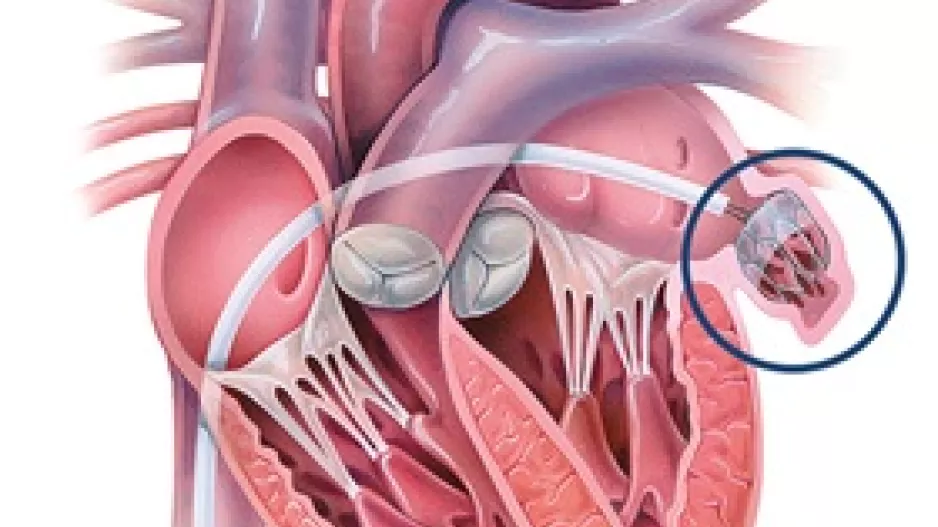
When patients with atrial fibrillation (AFib) require left atrial appendage occlusion (LAAO) to prevent their risk of stroke, the procedures are typically guided by transesophageal echocardiography (TEE). Intracardiac echocardiography (ICE) has…
A Rhode Island women has filed a lawsuit against Panera Bread that alleges she developed multiple heart conditions as a direct result of drinking the restaurant’s Charged Lemonade drinks.
NBC News explored the allegations in a new report…
Integer Holdings Corp., a Texas-based medical device outsource manufacturer, has finalized a deal to acquire Pulse Technologies, a privately-held contract manufacturing company, for approximately $140 million.
…
When patients with pacemakers die, what happens to the device? Typically, it ends up being discarded and forgotten—they were designed to be single-use devices, after all—but that does not have to be the case.
For several years now,…
Bruce Wilkoff, MD, a veteran cardiologist with Cleveland Clinic and a giant in the field of cardiovascular medicine, died Sunday, Jan. 7. He was 69 years old.
Wilkoff worked at Cleveland Clinic for 37 years, specializing in such areas as…
Treating patients with 10 days of colchicine therapy after they undergo catheter ablation for atrial fibrillation (AFib) does not lower their risk of arrhythmia recurrence, according to new research published in Circulation: Arrhythmia and…