More Articles
Performing left atrial appendage occlusion (LAAO) at the same time as other cardiovascular interventions does not increase the patient’s risk of major adverse events, according to new research published in JACC: Cardiovascular Interventions.[1]…
Abbott announced this week that more than 30 patients in Australia were treated with the company’s new pulsed field ablation (PFA) system, representing the first time the technology has been used to treat arrhythmias such as atrial fibrillation…
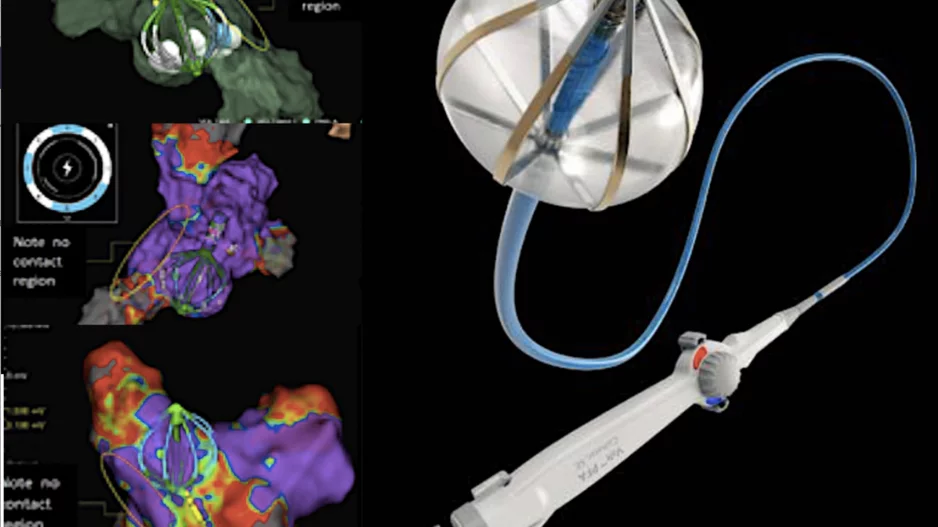
When patients with atrial fibrillation (AFib) require left atrial appendage occlusion (LAAO) to prevent their risk of stroke, the procedures are typically guided by transesophageal echocardiography (TEE). Intracardiac echocardiography (ICE) has…
A Rhode Island women has filed a lawsuit against Panera Bread that alleges she developed multiple heart conditions as a direct result of drinking the restaurant’s Charged Lemonade drinks.
NBC News explored the allegations in a new report…
Integer Holdings Corp., a Texas-based medical device outsource manufacturer, has finalized a deal to acquire Pulse Technologies, a privately-held contract manufacturing company, for approximately $140 million.
…
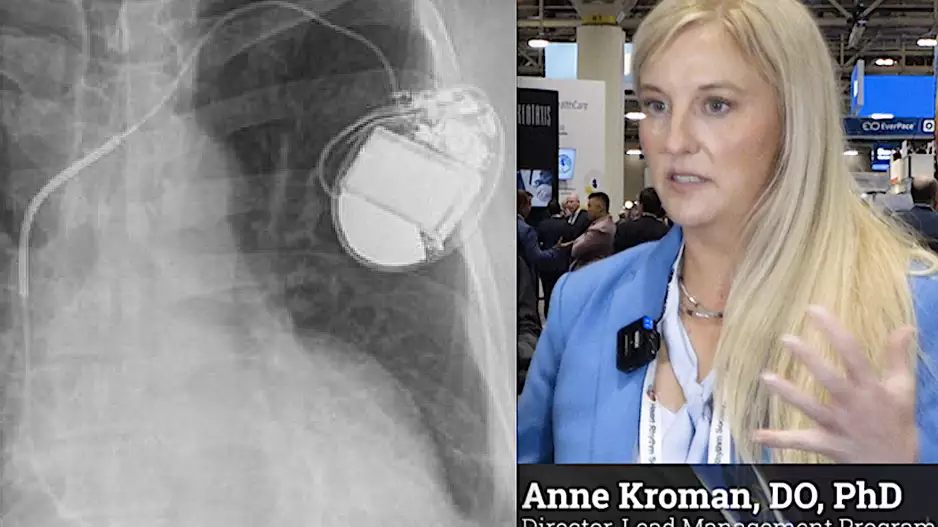
When patients with pacemakers die, what happens to the device? Typically, it ends up being discarded and forgotten—they were designed to be single-use devices, after all—but that does not have to be the case.
For several years now,…
Bruce Wilkoff, MD, a veteran cardiologist with Cleveland Clinic and a giant in the field of cardiovascular medicine, died Sunday, Jan. 7. He was 69 years old.
Wilkoff worked at Cleveland Clinic for 37 years, specializing in such areas as…
Treating patients with 10 days of colchicine therapy after they undergo catheter ablation for atrial fibrillation (AFib) does not lower their risk of arrhythmia recurrence, according to new research published in Circulation: Arrhythmia and…
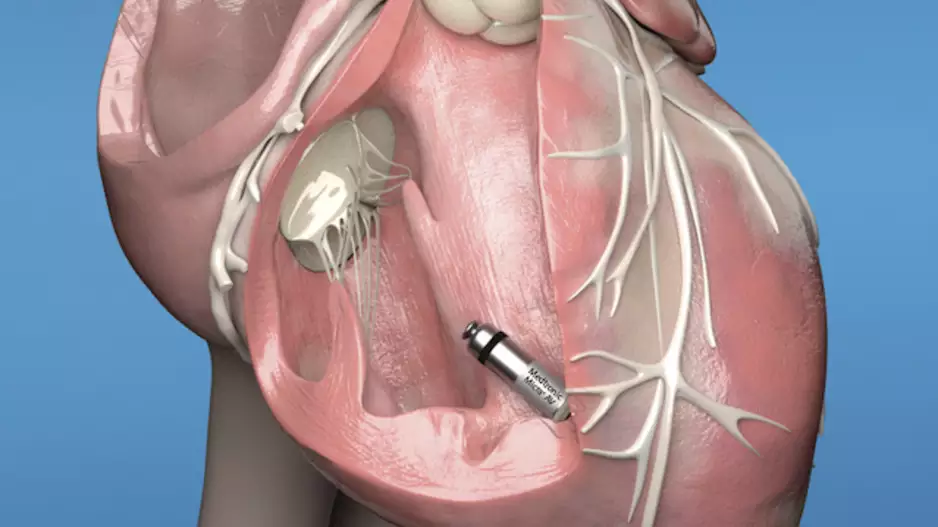
Medtronic has gained European CE mark approval for its next-generation Micra leadless pacemakers, the Micra AV2 and Micra VR2 transcatheter pacing systems. Both devices gained U.S. Food and Drug Administration…
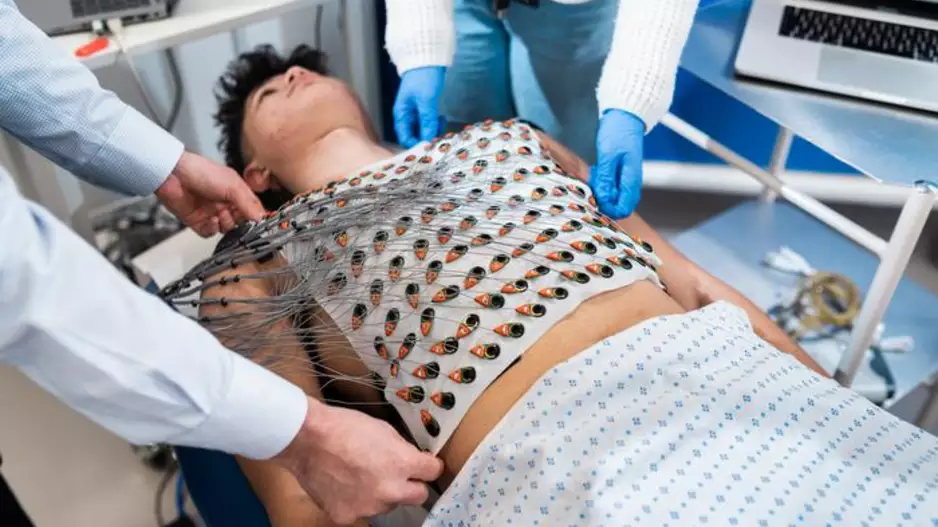
Researchers have developed a reusable vest capable of mapping the heart’s electrical activity in just five minutes, sharing their findings in the Journal of Cardiovascular Magnetic Resonance.[1]. The group—made up of cardiologists and…
The Food and Drug Administration (FDA) this week cleared a pulsed field ablation (PFA) system, the Medtronic PulseSelect System, for the treatment of both paroxysmal and persistent atrial fibrillation (AF). The approval is the first for this…
Transcatheter aortic valve replacement (TAVR) patients face a heightened risk of adverse short- and long-term outcomes if they present with preexisting…
Ajax Health, a California-based venture capital firm, has launched a new healthcare company focused on atrial fibrillation (AFib) ablation technology. The new entity, Cortex, has already raised $90 million in funding as it enters the market.…
The American College of Cardiology (ACC) and American Heart Association (AHA) just published…
The American College of Cardiology (ACC) and American Heart Association (AHA) have published new guidelines for the diagnosis and management of atrial fibrillation (AFib).…
The bodies of organ donors are regularly treated with thyroid hormones to preserve heart function and ensure any transplant procedures are a success. However, a new study published in The New England Journal of Medicine suggests those…
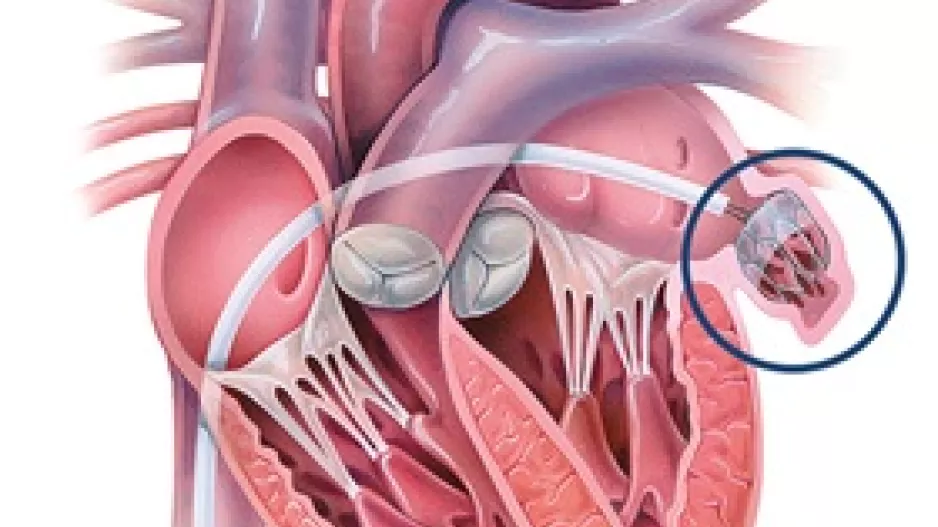
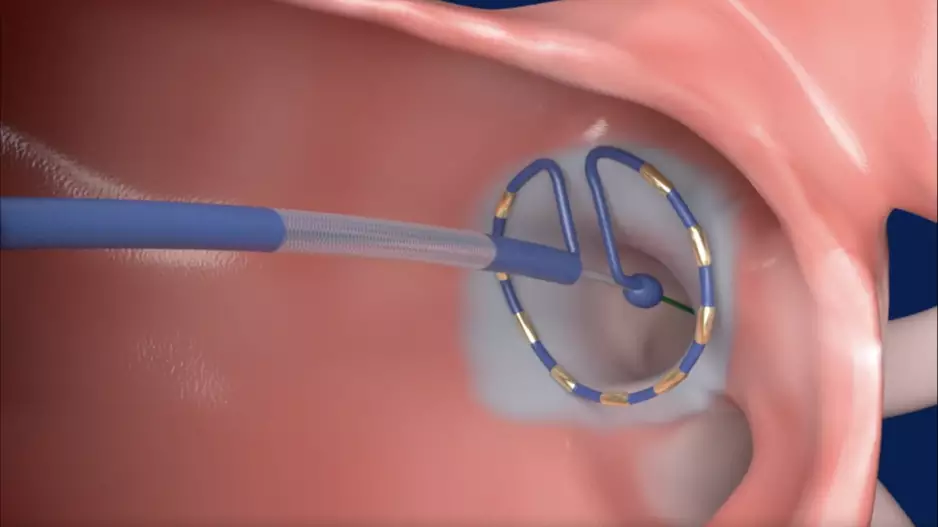
Johnson & Johnson MedTech has acquired Laminar, a California-based healthcare technology company known for its investigational left atrial appendage (LAA) elimination technology, for $400 million. The deal could also include additional…
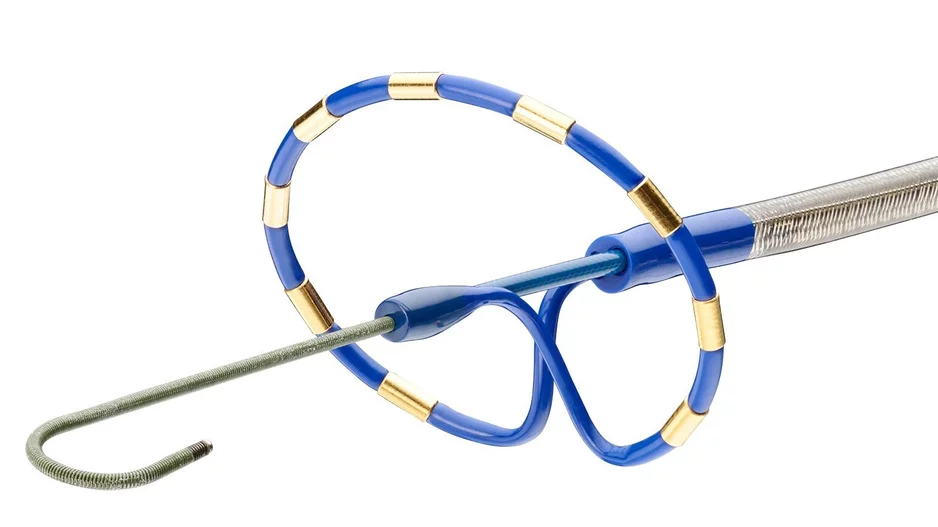
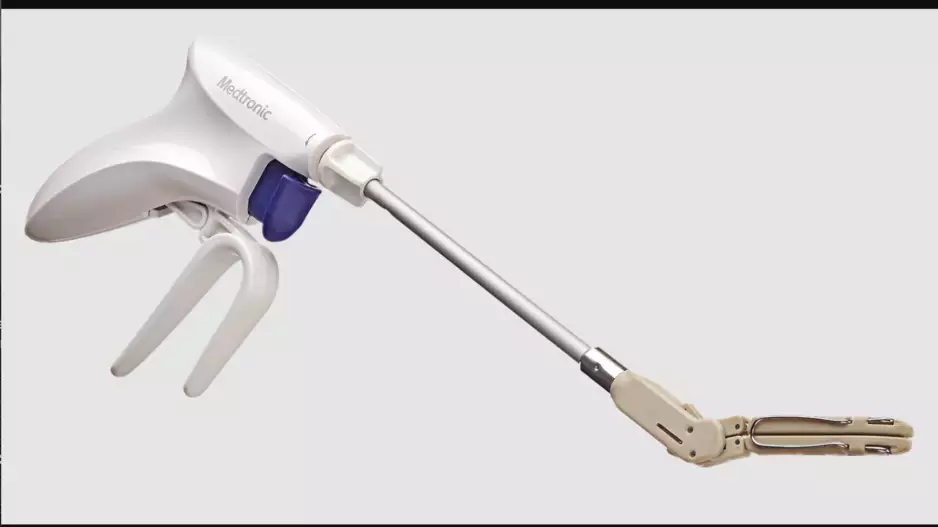
Medtronic has added a new device, the Penditure Left Atrial Appendage (LAA) Exclusion System, to its cardiac surgery portfolio. The device was designed for the exclusion of the LAA in patients undergoing concomitant heart surgeries, a step…
Bayer has ended a clinical trial for its new atrial fibrillation (AFib) drug much earlier than planned based on recommendations from the Independent Data Monitoring Committee (IDMC). OCEANIC-…
Medtronic has gained European CE mark approval for its PulseSelect Pulsed Field Ablation (PFA) System and the Nitron CryoConsole.
PulseSelect, designed to treat atrial fibrillation (AFib), is a key piece of Medtronic’s cardiac ablation…