More Articles
Eko Health has received a new Category III CPT code from the American Medical Association (AMA) for its Sensora platform, ensuring its use can be properly documented by coding specialists whenever necessary…
It may be impossible to accurately predict when heart attack survivors are going to experience sudden cardiac death later in life, according to a new analysis published in European Heart Journal.[1] That could change in the future, of…
Treating atrial fibrillation (AFib) during cardiac surgeries with concomitant surgical ablation (SA) is associated with better long-term patient outcomes, according to a new meta-analysis published in the American Journal of Cardiology.[…
Johnson & Johnson MedTech has received U.S. Food and Drug Administration (FDA) approval for its Varipulse pulsed field ablation (PFA) system designed to treat paroxysmal atrial fibrillation (AFib). The…
Boston Scientific has agreed to acquire Cortex, a healthcare technology company launched by the venture capital firm Ajax Health in 2023, to expand its electrophysiology (…
The Heart Rhythm Society (HRS) has established a new advocacy group dedicated to lobbying for causes important to both electrophysiologists and the heart patients they treat on a daily basis.
…
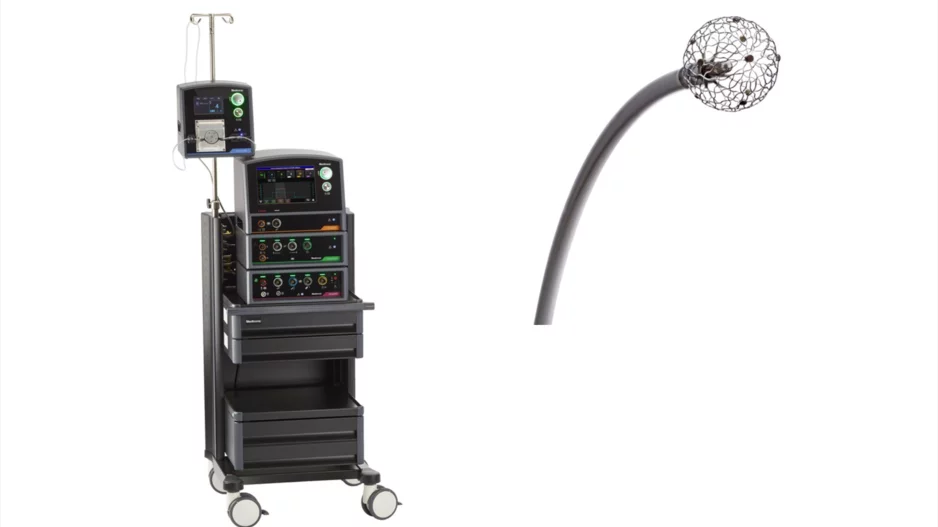
Medtronic has received U.S. Food and Drug Administration (FDA) approval for its new Affera mapping and ablation system with the Sphere-9 catheter, an all-in-one electrophysiology offering capable of pulsed field…
Boston Scientific has received U.S. Food and Drug Administration (FDA) approval for its Farawave Nav ablation…
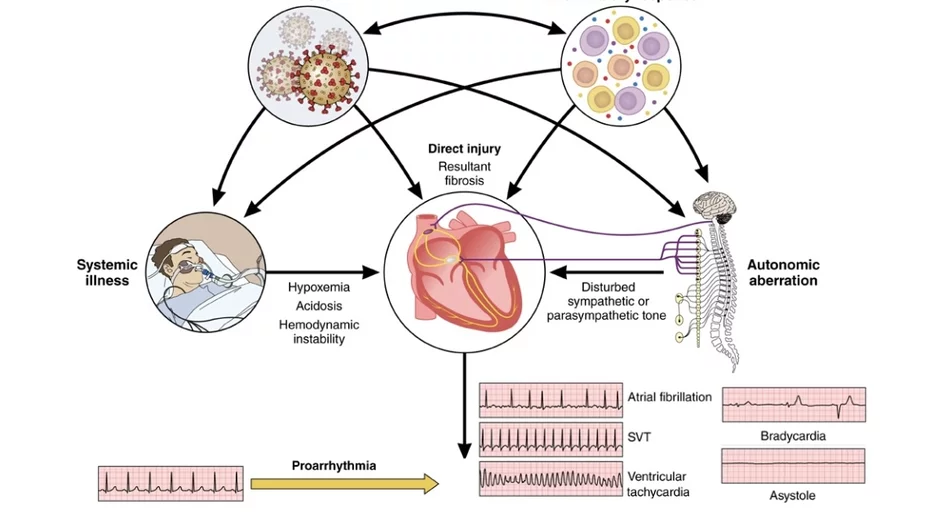
Cardiac arrhythmias are common in patients with COVID-19 infections and during recovery, which prompted a new scientific statement from the…
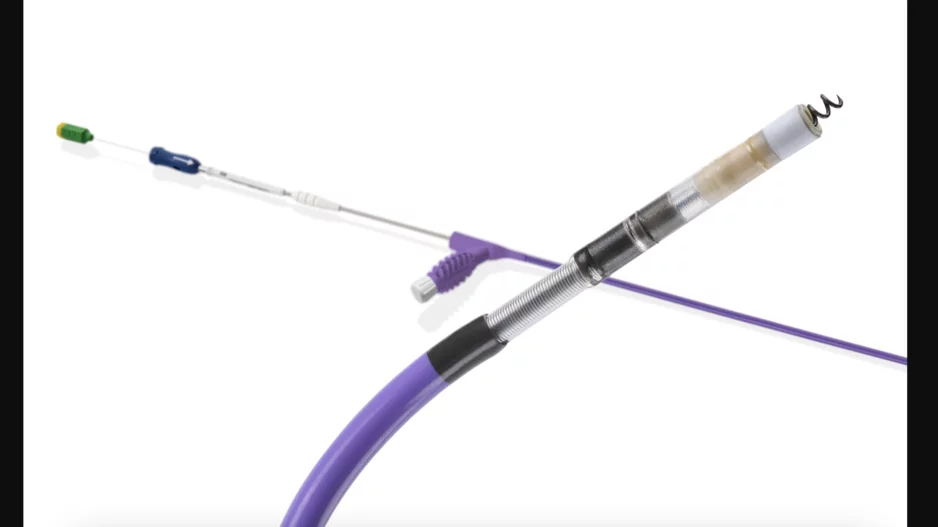
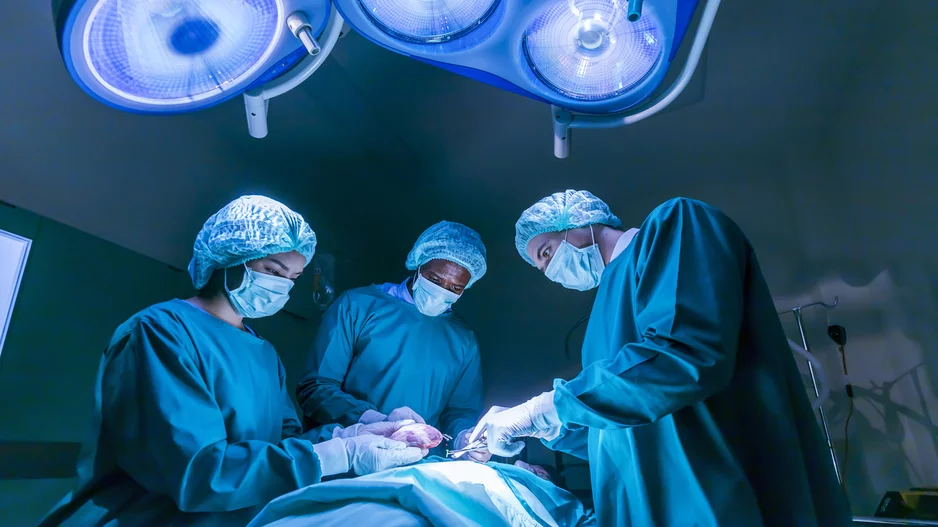
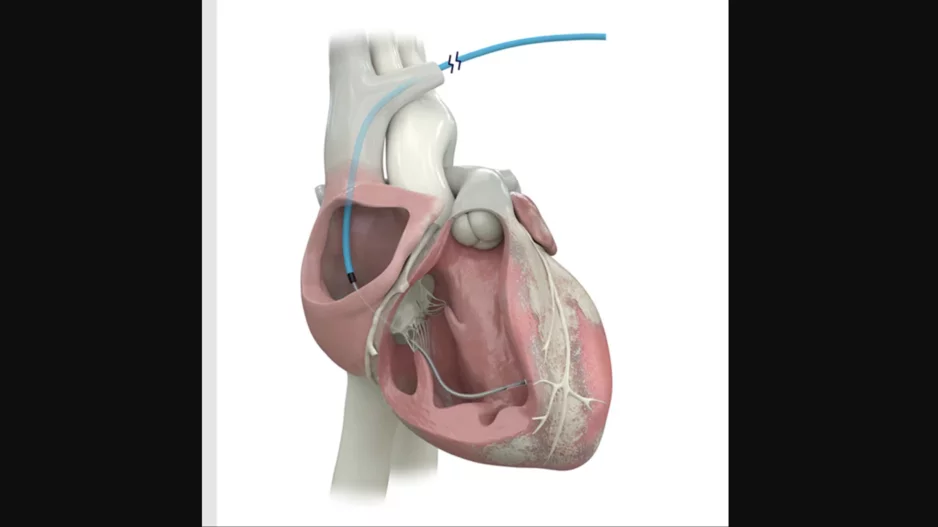
Cardiologists have performed what they believe to be the world’s first substernal lead extraction, sharing their experience in JACC: Case Reports.[1]
The care team, working out of the Regional University Hospital Center of Tours in…
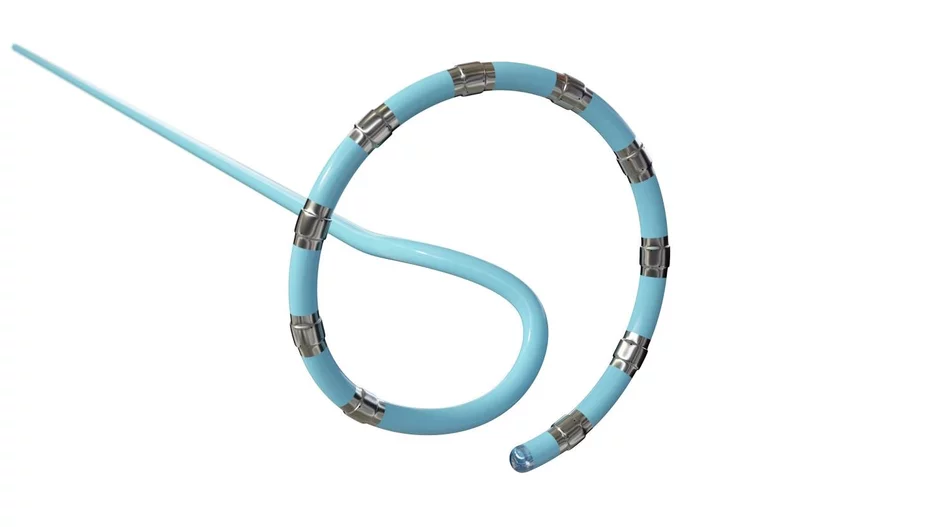
Medtronic’s OmniaSecure defibrillation lead, already associated with positive early outcomes, is projected to deliver long-term durability, according to a new analysis published in HeartRhythm.[1]
The device, which Medtronic…
Patient care in the United States has never been more advanced than it is today, with artificial intelligence and other emerging technologies evolving at a remarkable…
The Heart Rhythm Society (HRS) has shared a new statement emphasizing the importance of atrial fibrillation (AFib) ablation procedures being performed by trained electrophysiologists (EPs).
These…
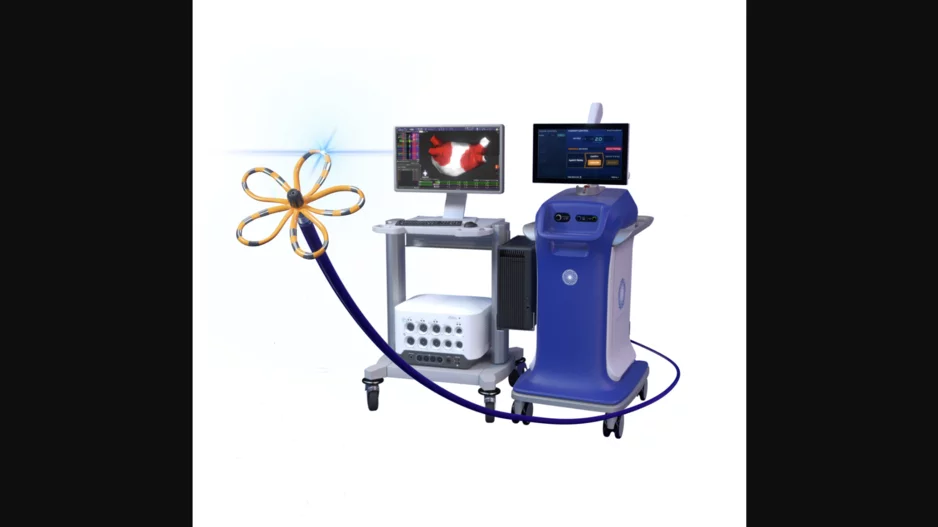
Focal pulsed field ablation (PFA) is a safe, effective treatment option for premature ventricular contractions (PVC), according to new data published in Circulation: Arrhythmia and Electrophysiology.[1]
“Limited evidence on PFA-…
Heart tissue samples that spent 30 days at the International Space Station (ISS) showed low gravity conditions in space weakened the tissues and disrupted their normal rhythmic beats when compared to earth-bound samples from the same source. The…
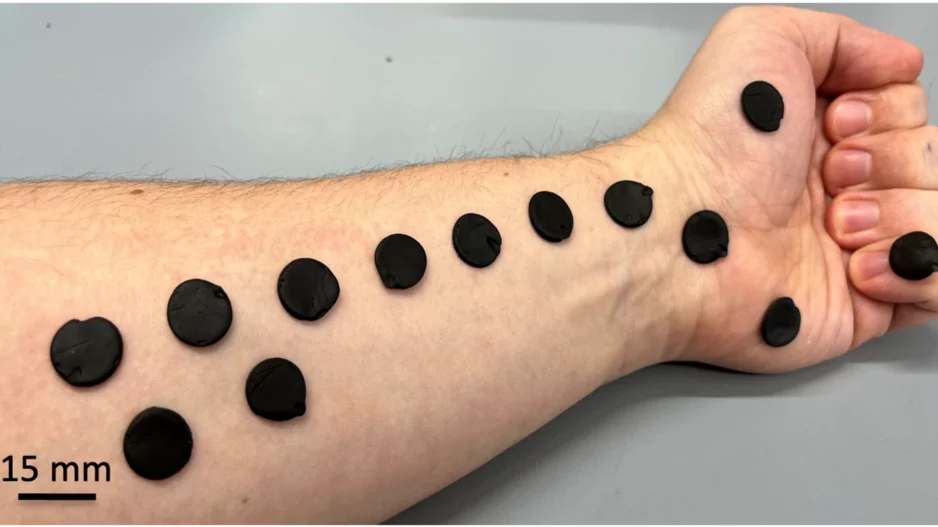
Researchers have found that homemade play-putty can effectively capture electrocardiogram (ECG) signals at a level comparable to commercially…
Left bundle branch area pacing (LBBAP) is associated with better long-term outcomes than traditional right ventricular pacing (RVP) when patients require a permanent pacemaker after…
Boston Scientific has received U.S. Food and Drug Administration (FDA) approval for its Ingevity+ pacing leads to be used for left bundle branch area pacing (LBBAP) when connected to a single- or dual-chamber…
Two U.S. healthcare technology companies have announced a significant transaction that could shake up the country’s electrophysiology market.
Merit Medical Systems, a Utah-based medical device…
Biotronik has received U.S. Food and Drug Administration (FDA) approval to make its Solia S lead and Selectra 3D catheter available for a new indication: left bundle branch area pacing (LBBAP).
LBBAP…
Performing low-voltage-area (LVA) ablation after pulmonary vein isolation (PVI) when treating persistent atrial fibrillation (AFib) does not appear to improve outcomes, according to a new analysis presented at…




![Cardiologists have performed what they believe to be the world’s first substernal lead extraction, sharing their experience in JACC: Case Reports.[1]The device being extracted, Medtronic’s Aurora EV-ICD, received U.S. Food and Drug Administration (FDA) approval in October 2023.](/sites/default/files/styles/media_image/public/2024-10/screenshot_2024-10-11_at_11.56.29_am.png.webp?h=6be7584a&itok=Po6DYlmC)
![Focal pulsed field ablation (PFA) is a safe, effective treatment option for premature ventricular contractions (PVC), according to new data published in Circulation: Arrhythmia and Electrophysiology.[1]](/sites/default/files/styles/media_image/public/2024-09/screenshot_2024-09-25_at_1.12.29_pm.png.webp?h=6be7584a&itok=okd2xvmf)