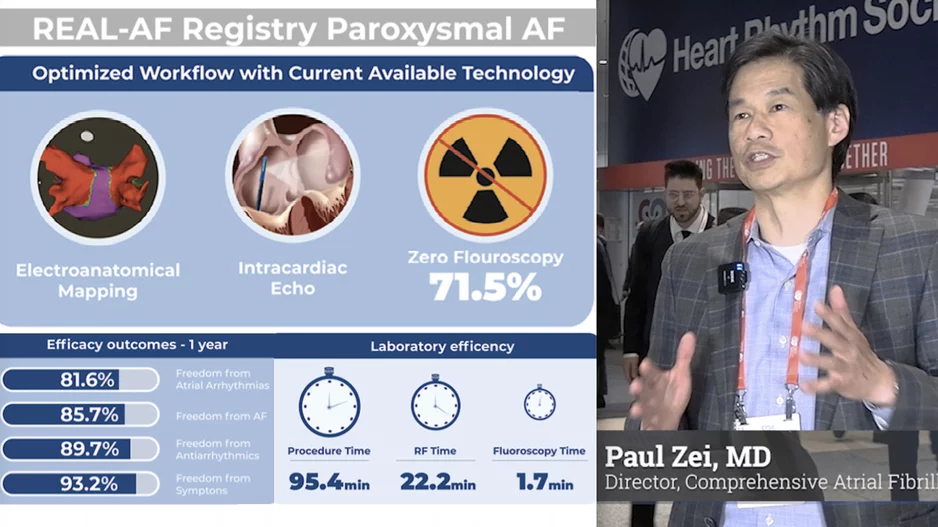
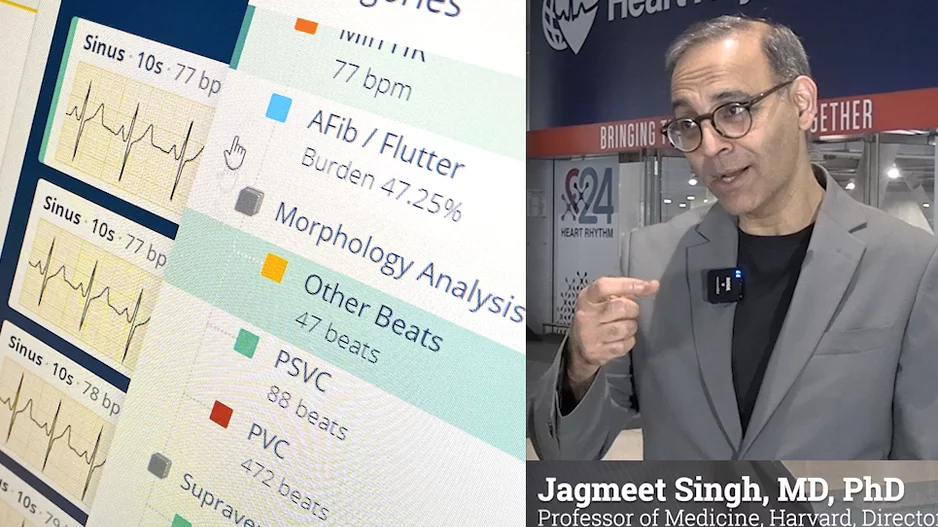
More Articles
Asking pharmacists to play a more active role in patient care can help patients with undiagnosed and undertreated atrial fibrillation (AFib) receive the care they need, according to a new study published in JAMA Network Open.[1]
“…
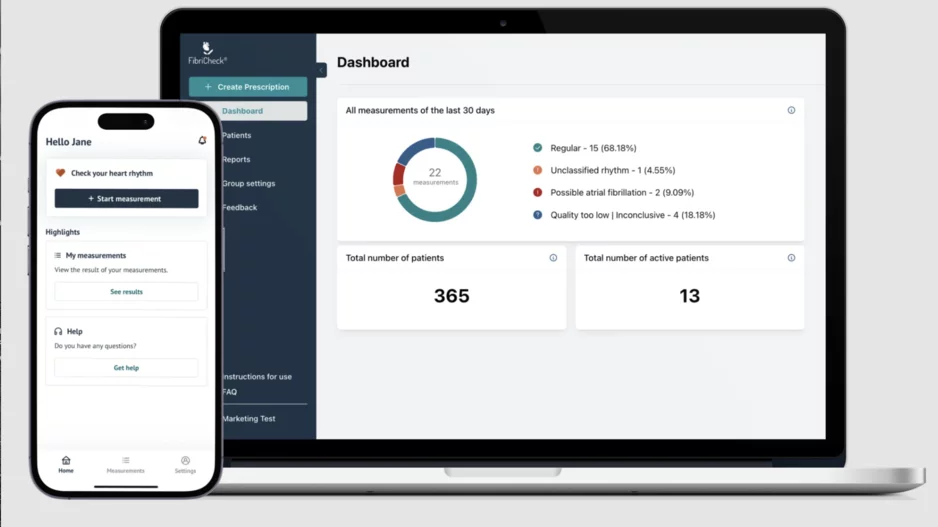
FibriCheck, a Belgium-based healthcare technology company, has gained U.S. Food and Drug Administration (FDA) for its artificial…
Volta Medical, a French healthcare company focused on improving care for atrial fibrillation (AFib) patients, has agreed to integrate its artificial intelligence (AI)…
When patients require a permanent pacemaker (PPM) after transcatheter aortic valve replacement (TAVR), should care teams turn to a transvenous or…
Cardiology salaries have continued to climb in 2024, according to a new survey from the American Medical Group Association (AMGA). Among general cardiologists, for example, median compensation jumped nearly 8%…
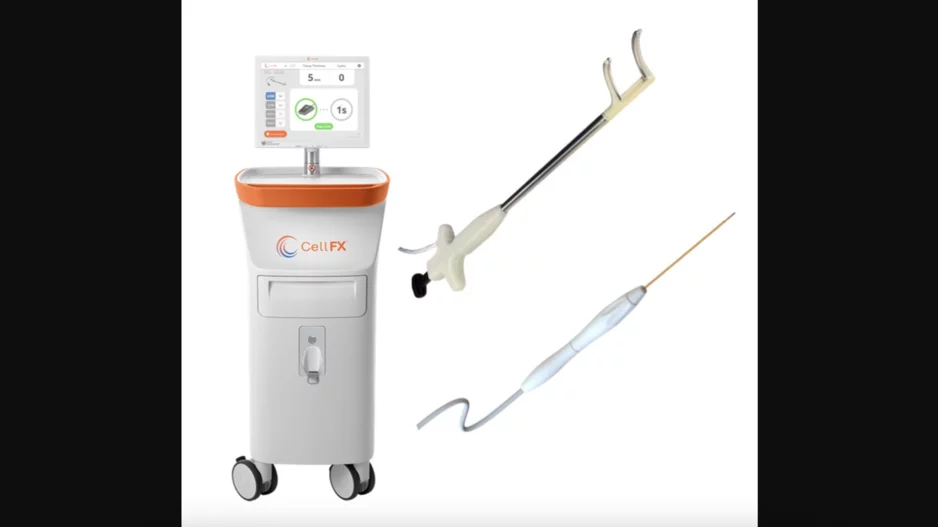
Pulse Biosciences, a Florida-based healthcare technology company, has received the FDA’s breakthrough device designation for its new CellFX Nanosecond Pulsed Field Ablation (PFA) technology.
According to Pulse Biosciences, its…
Pulsed field ablation (PFA) has gained significant momentum as a treatment option for atrial fibrillation (AFib) in recent months, with both…
Medicare Part B enrollees will pay significantly less for 64 different prescription medications for the next three months, according to a new announcement from the U.S. Department of Health and Human Services (HHS…
Chicago-based Tempus AI has gained clearance from the U.S. Food and Drug Administration (FDA) for a new artificial intelligence (AI)…
Patients with persistent atrial fibrillation (AFib) who undergo transcatheter aortic valve replacement (TAVR) may face a heightened risk of long-term…
Energy drinks may increase the risk of sudden cardiac arrest (SCA) in people with underlying heart disease, according to a new analysis published in Heart Rhythm.[1]
It is too early to know for sure, researchers wrote, but the…
Medicalgorithmics, a Polish healthcare technology company focused on using advanced artificial intelligence (AI) models to diagnose arrhythmias, has received approval…
Elutia, a Maryland-based healthcare technology company, has received clearance from the U.S. Food and Drug Administration (FDA) for EluPro, its new biologic envelope specifically designed for patients with…
Kardium, a Canadian medical device company focused on electrophysiology technologies, has raised $104 million in new financing. Fidelity Management and Research Company led the funding round; T. Rowe Price Associates and new investor Durable…
The U.S. Food and Drug Administration (FDA) is warning Americans not to “eat, sell or serve” mushroom-laced chocolates sold by Diamond Shruumz. Sickness and hospitalizations after eating these products have been…
Stuart Connolly, MD, a veteran cardiologist known for his groundbreaking work in the field of electrophysiology, died June 2 after a long battle with neuroendocrine cancer. He was 75 years old.
Connolly spent 40 years at McMaster…