More Articles
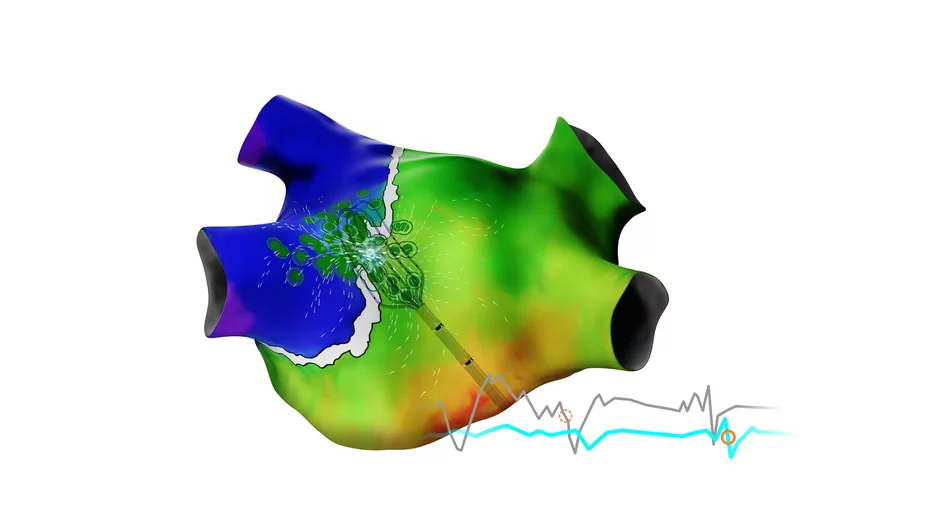
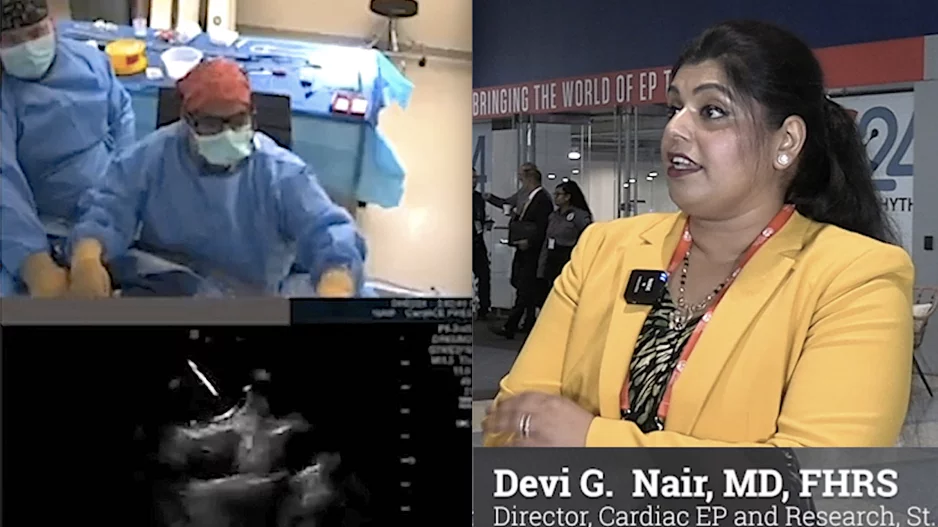
A new pulsed field ablation (PFA) system from Kardium could give cardiologists a new tool in the war against atrial fibrillation (AFib), according to new data published in Heart Rhythm.[1]
The…
WearLinq, a San Francisco-based healthcare technology company, has acquired AMI Cardiac Monitoring, an independent diagnostic testing facility, to help increase the reach of its…
The late-breaking U.S. multicenter admIRE clinical trial at the Heart Rhythm Society 2024 meeting, showed positive data on the long-term safety and effectiveness of the the Biosense Webster Varipulse…
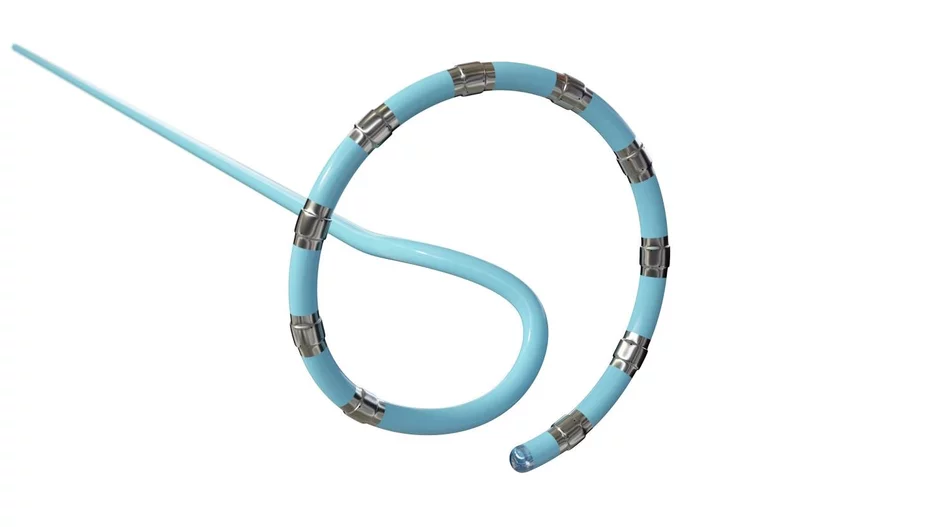
Atraverse Medical, a San Diego-based medical device company focused on left-heart technology, has received U.S. Food and Drug Administration (FDA) clearance for its Hotwire radiofrequency (RF) guidewire.
…Medtronic has added new artificial intelligence (AI) capabilities to its Reveal LINQ insertable cardiac monitor (ICM), the company announced Monday, May 13. The…
…
A total of 21 late-breaking science presentations are scheduled for Heart Rhythm 2024, the annual meeting of the Heart Rhythm Society (HRS). The meeting will take place May 16-19 in Boston.
…
Biosense Webster, a part of Johnson & Johnson MedTech, has released the latest version of its Carto 3 3D mapping software for cardiac ablation procedures. The update, officially known as Carto 3 System Version 8, includes new modules designed…
Apple Watch AFib feature becomes first-ever digital tool approved by FDA to evaluate medical devices
The Apple Watch’s atrial fibrillation (AFib) history feature has qualified for the FDA’s Medical Device Development Tools (MDDT) program, meaning it can now…
Catheter ablation is known to benefit patients who present with atrial fibrillation (AFib) and heart failure with reduced ejection fraction (HFrEF). Its potential impact on patients with AFib and heart failure with preserved ejection fraction (…
Atrial fibrillation (AFib) is one of the most common heart complications in the United States, but many AFib patients fail to take the medications prescribed by their cardiologists. The American Heart Association…
Heart failure is the most common cardiovascular complication among patients with atrial fibrillation (AFib), according to new data published in The BMJ.[1] This may be a surprise to many, researchers said, because of how much attention…
Lexeo Therapeutics is one step closer to receiving approval from the U.S. Food and Drug Administration (FDA) for its new drug candidate for Friedreich's ataxia (FA) cardiomyopathy.
The New York City-…
Tecarfarin, a new anticoagulant from Cadrenal Therapeutics, has received the FDA’s orphan drug…
Biosense Webster, part of Johnson & Johnson MedTech, has submitted a premarket approval application for its Varipulse pulsed field ablation (PFA) system to…



![Abbott’s Aveir DR leadless pacemaker, the world’s very first dual-chamber pacing solution of its kind, is associated with a “reliable” performance after six months, according to new data published in Heart Rhythm.[1]](/sites/default/files/styles/media_image/public/2024-05/screen_shot_2024-05-10_at_11.33.50_am.png.webp?h=3af9dcd6&itok=ZuCFBzfX)