More Articles
The first modular, leadless cardiac rhythm management (CRM) system composed of a leadless pacemaker and a subcutaneous, leadless implantable cardioverter defibrillator (ICD) was linked to positive early results at…
PaceMate, a Florida-based company focused on remote monitoring and patient management technologies, has acquired Medtronic’s…
The U.S. Food and Drug Administration (FDA) has announced that Defibtech, a Nihon Kohden company, is recalling its RMU-2000 ARM XR Chest Compression Device due to significant safety concerns. This is a Class I…
Left atrial appendage occlusion (LAAO) with the Watchman FLX device from Boston Scientific is associated with positive outcomes and limited adverse events after one year, according to new findings published in Circulation: Cardiovascular…
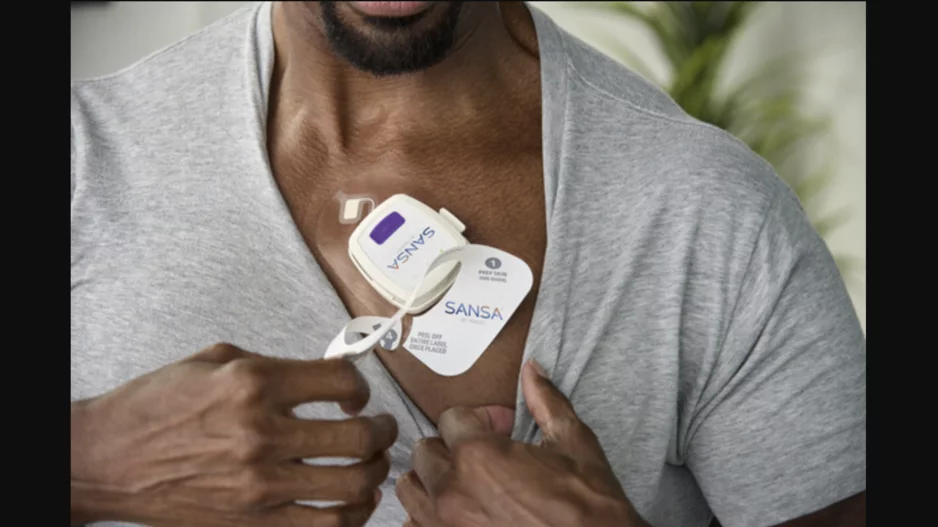
Huxley Medical, an Atlanta-based medical device company, has secured U.S. Food and Drug Administration (FDA) clearance for its new Sansa device, a chest-worn patch…
Treating mitral regurgitation with transcatheter mitral edge-to-edge repair (TEER) using the MitraClip device is associated with a low risk of…
How can cardiologists limit ischemic strokes in patients with atrial fibrillation (AFib) following a successful transcatheter aortic valve replacement (…
Asking pharmacists to play a more active role in patient care can help patients with undiagnosed and undertreated atrial fibrillation (AFib) receive the care they need, according to a new study published in JAMA Network Open.[1]
“…
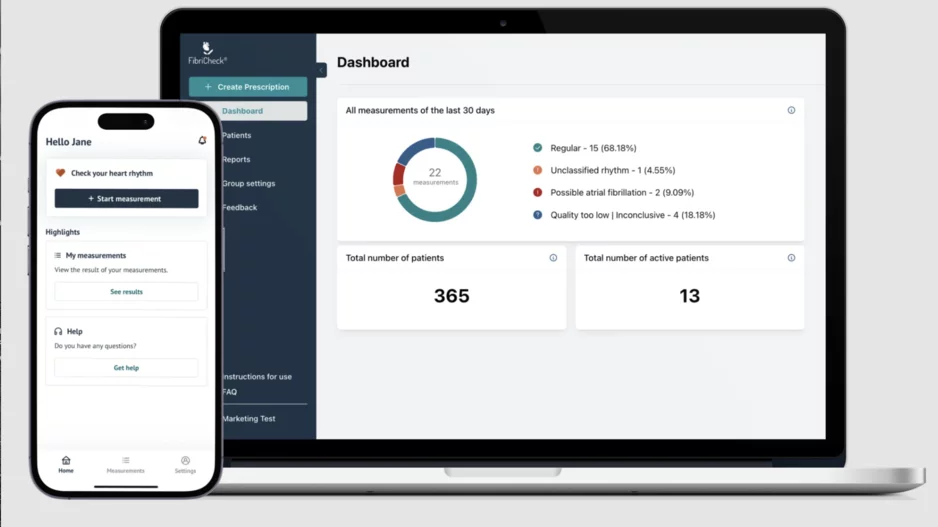
FibriCheck, a Belgium-based healthcare technology company, has gained U.S. Food and Drug Administration (FDA) for its artificial…
Volta Medical, a French healthcare company focused on improving care for atrial fibrillation (AFib) patients, has agreed to integrate its artificial intelligence (AI)…
When patients require a permanent pacemaker (PPM) after transcatheter aortic valve replacement (TAVR), should care teams turn to a transvenous or…
Cardiology salaries have continued to climb in 2024, according to a new survey from the American Medical Group Association (AMGA). Among general cardiologists, for example, median compensation jumped nearly 8%…
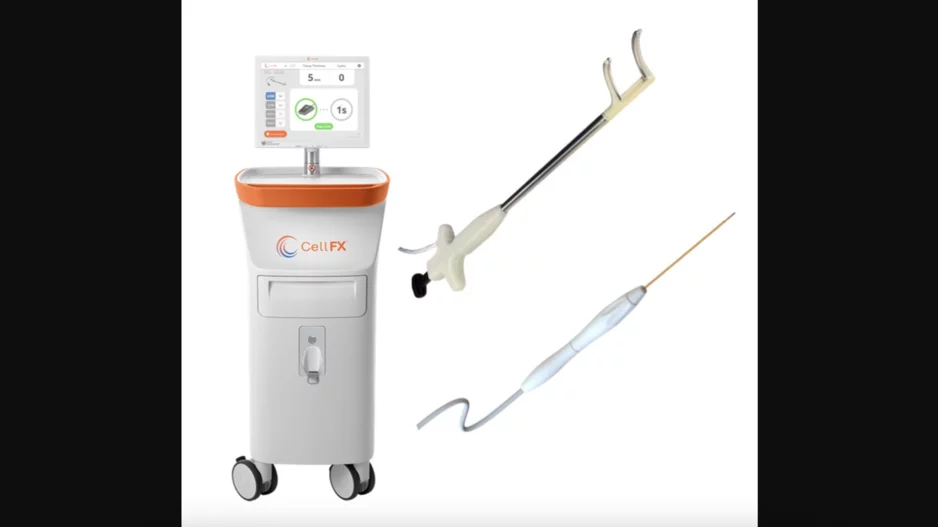
Pulse Biosciences, a Florida-based healthcare technology company, has received the FDA’s breakthrough device designation for its new CellFX Nanosecond Pulsed Field Ablation (PFA) technology.
According to Pulse Biosciences, its…
Pulsed field ablation (PFA) has gained significant momentum as a treatment option for atrial fibrillation (AFib) in recent months, with both…
Medicare Part B enrollees will pay significantly less for 64 different prescription medications for the next three months, according to a new announcement from the U.S. Department of Health and Human Services (HHS…
Chicago-based Tempus AI has gained clearance from the U.S. Food and Drug Administration (FDA) for a new artificial intelligence (AI)…
Patients with persistent atrial fibrillation (AFib) who undergo transcatheter aortic valve replacement (TAVR) may face a heightened risk of long-term…
Energy drinks may increase the risk of sudden cardiac arrest (SCA) in people with underlying heart disease, according to a new analysis published in Heart Rhythm.[1]
It is too early to know for sure, researchers wrote, but the…
Medicalgorithmics, a Polish healthcare technology company focused on using advanced artificial intelligence (AI) models to diagnose arrhythmias, has received approval…

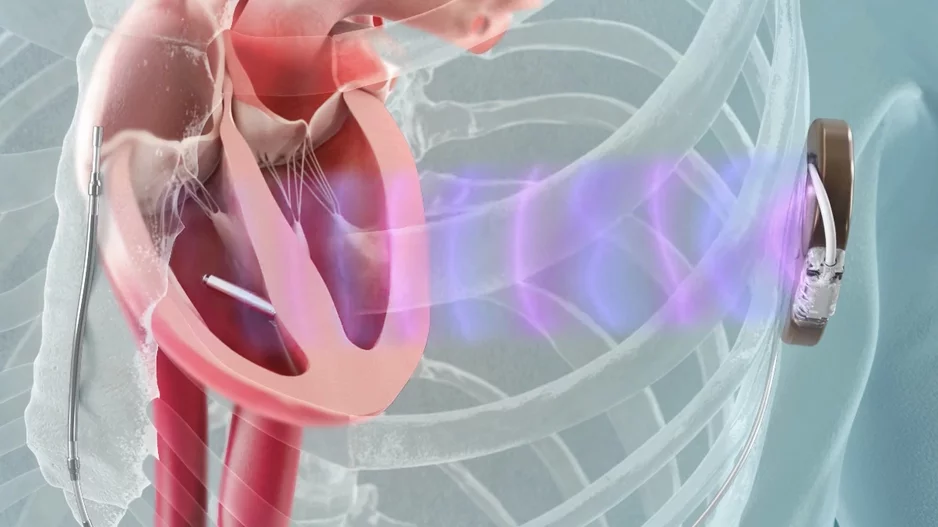


![Left atrial appendage occlusion (LAAO) with the Watchman FLX device from Boston Scientific is associated with positive outcomes and limited adverse events after one year, according to new findings published in Circulation: Cardiovascular Interventions.[1] Many prior Watchman FLX studies, including PINNACLE FLX, had focused on the device’s performance in a controlled setting. The study’s authors hoped to gain a better understanding of its real-world impact by reviewing registry data from more than 97,000 U.S](/sites/default/files/styles/media_image/public/2024-08/screenshot_2024-08-12_at_11.35.13_am.png.webp?h=6be7584a&itok=djouoZ9Q)
![Treating mitral regurgitation with transcatheter mitral edge-to-edge repair (TEER) using the MitraClip device is associated with a low risk of cerebrovascular accidents (CVAs) such as stroke and transient ischemic attack (TIA), according to new data published in The American Journal of Cardiology.[1]](/sites/default/files/styles/media_image/public/2024-08/small-tech-big-impact-mitraclip-960x430.jpg.webp?h=658ac4ee&itok=0Rn0kdHz)