More Articles
Massachusetts-based Zoll has received U.S. Food and Drug Administration (FDA) approval for its Zenix defibrillator-monitor for EMS and hospital use. The device includes new…
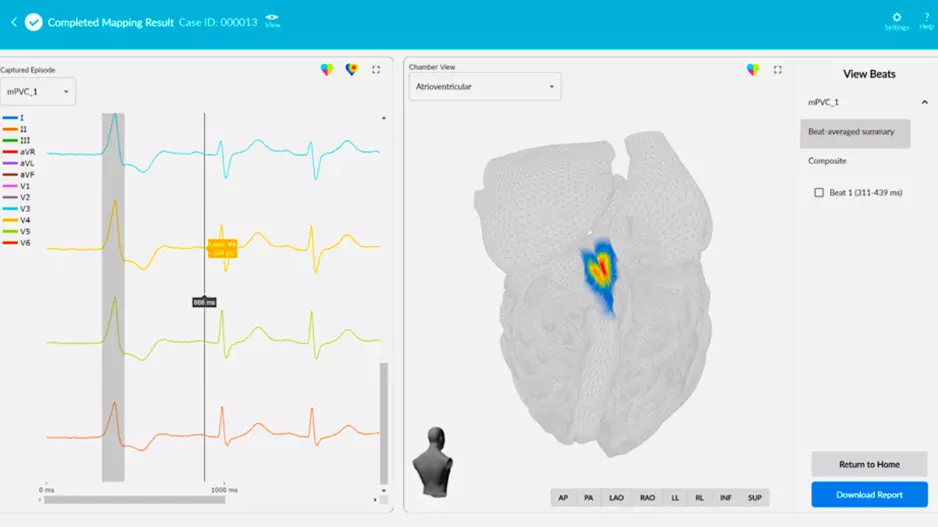
San Diego-based Vektor Medical has gained CE mark approval for the vMap System, which uses artificial intelligence (AI) to turn 12-lead electrocardiograms (ECGs) into…
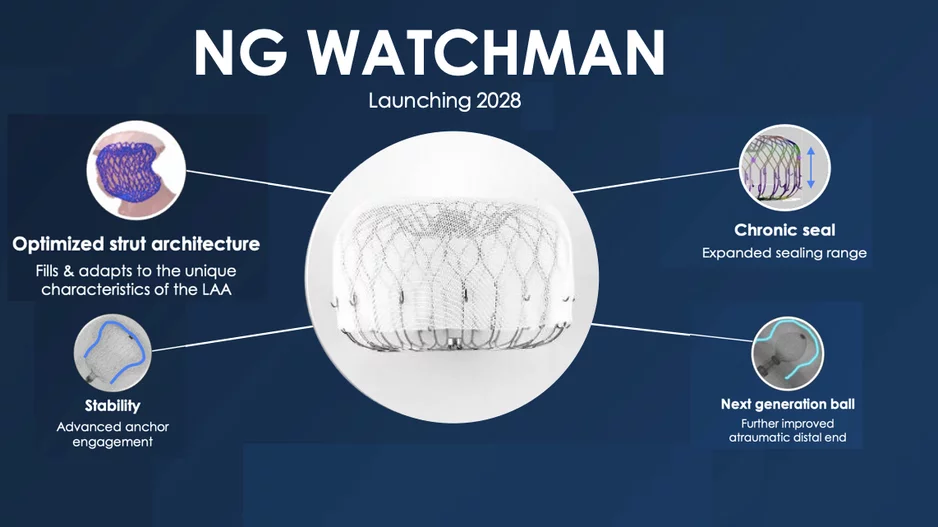
Boston Scientific announced at its Investor Day event Sept. 30 it plans to launch its fourth-generation Watchman left atrial appendage closure (LAAC) device, which will…
The use of augmented reality (AR) during cardiac ablation procedures may be improved by adding “virtual screens” that clinicians can customize as they see fit. That was the primary takeaway of a new analysis published in the Journal of…
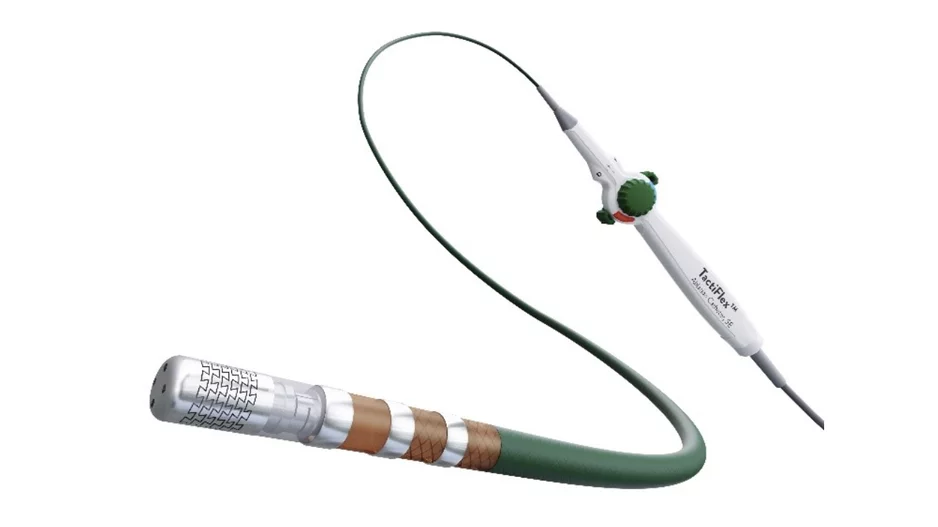
Abbott has issued a warning on the proper handling of its TactiFlex Ablation Catheter, Sensor Enabled,…
A safety issue with certain Boston Scientific defibrillation leads has resulted in a series of new Class I recalls, according to the U.S. Food and Drug Administration (FDA). Unlike some recalls, these do not…
Boston Scientific has agreed to acquire Elutia’s drug-eluting envelope business for $88 million. The cash transaction is expected to close in late 2025.
Elutia is the Maryland-based medtech company behind…
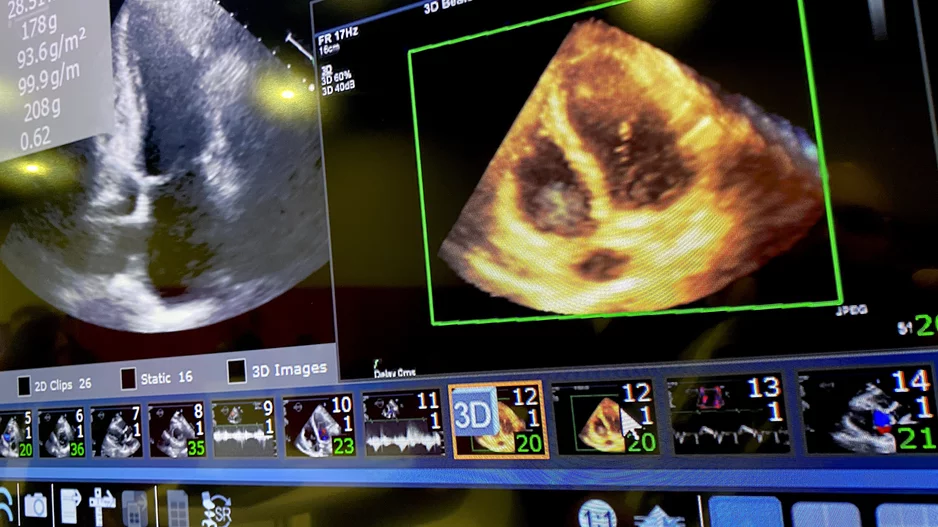
Evaluating left ventricular diastolic function (LVDF) with echocardiography can help identify individuals at an increased risk of developing atrial fibrillation (AFib), according to new findings presented at the American…
In July, the Centers for Medicare and Medicaid Services (CMS) proposed adding procedure codes for cardiac ablations to the Ambulatory Surgical Center (ASC) Covered Procedures List as part of the…
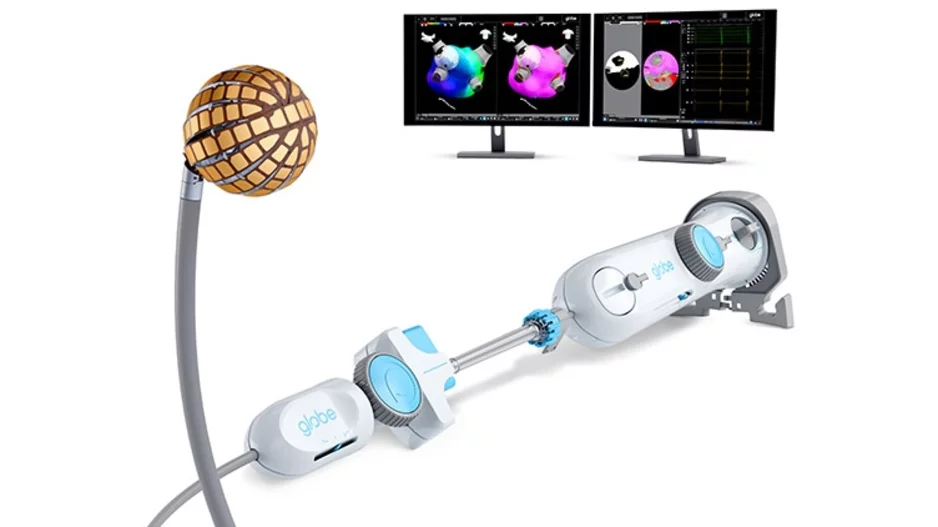
The U.S. Food and Drug Administration (FDA) has granted pre-market approval (PMA) for the Globe Pulsed Field Ablation (PFA) System, along with 510(k) clearance for both the Globe introducer sheath and the Globe Pulsed Field System mapping…
Philips introduced a new cardiac monitoring telemetry platform designed to address critical challenges in healthcare, including staff shortages and alarm management. A key component of the solution is the next-generation…
There has been a movement in electrophysiology (EP) labs to adopt high-density mapping catheters that allow more detailed information on the electrical activity in…
Pulsed field ablation (PFA) with Johnson & Johnson MedTech’s Varipulse platform is safe and effective, according to new real-world data presented at ESC Congress 2025…
Phrenic nerve injury (PNI) may still be a significant concern following pulsed field ablation (PFA) for atrial fibrillation (AFib), according to a new single-center study published in Heart Rhythm.[1]
“PNI is a known complication…
Medline ReNewal, Medline’s medical device reprocessing program, has announced that certain electrophysiology catheters should be removed from the market and returned to the manufacturer due to a risk that they may contain “small residual…
Boston Scientific’s Watchman device is associated with a heightened risk of air embolism events if the implant procedure is performed without positive pressure-controlled ventilation,…
The U.S. Food and Drug Administration (FDA) is sharing additional details about a safety issue with certain Boston Scientific defibrillation leads.
According to the agency, some of the company’s…
Coronary computed tomography angiography (CCTA) is more effective than transesophageal echocardiography (TEE) when it comes to detecting residual leaks following…
The Society for Cardiovascular Angiography and Interventions (SCAI) and Heart Rhythm Society (HRS) have published new evidence-based guidelines designed to bring more…
Early rhythm control (ERC) is still an effective treatment for atrial fibrillation (AFib) when patients also present with obesity or diabetes, according to a new analysis published in JAMA Cardiology.[1]
The international…
Giving colchicine to patients before transcatheter aortic valve replacement (TAVR) may reduce the risk of certain conduction disturbances, according to new research…