More Articles
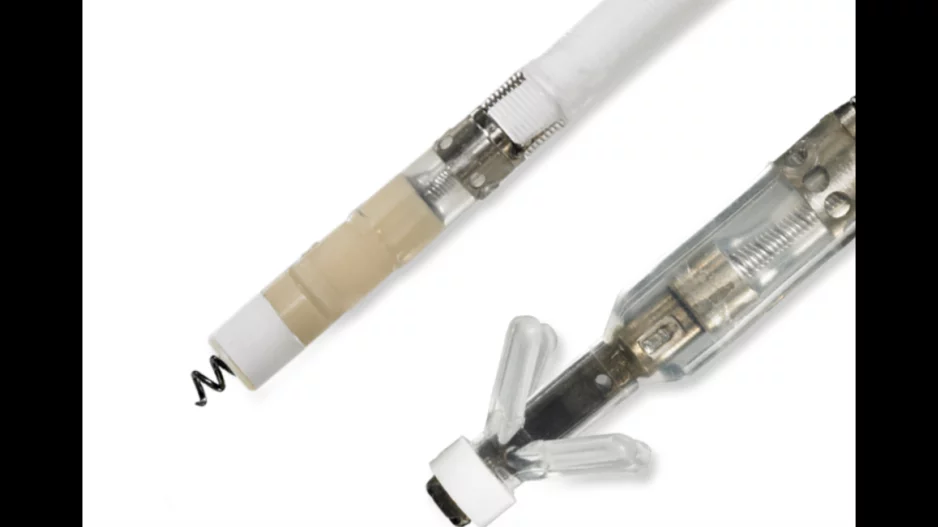
Boston Scientific is warning healthcare providers about the potential of rising low-voltage shock impedance (LVSI) with some of its single- and double-coil Reliance defibrillation leads coated with expanded polytetrafluoroethylene (ePTFE). This…
Patients may experience a significant amount of stress and/or anxiety due to receiving an implantable cardioverter-defibrillators (ICD), according to a new analysis published in The American Journal of Cardiology.[1] Can anything be done…
Stereotaxis, a St. Louis-based medtech company, has received U.S. Food and Drug Administration (FDA) clearance for the first robotically navigated, high-density…
Researchers had hoped a new drug targeting inflammation may be an effective treatment for atrial fibrillation (AFib). However, they found that the drug was actually associated with an increased AFib risk as opposed to a reduced risk. The group…
Solid Biosciences, a Massachusetts-based pharmaceutical company, has received the FDA’s fast track designation for a first-in-class gene therapy for the treatment of catecholaminergic polymorphic ventricular tachycardia (CPVT).
CPVT…
Royal Philips has launched the Philips ECG AI Marketplace, which gives cardiac care teams access to multiple vendor offerings in one central location. The company hopes this can help clinicians efficiently manage and implement artificial…
The U.S. Food and Drug Administration (FDA) has approved an updated irrigation flow rate for Johnson & Johnson MedTech’s Varipulse pulsed field ablation (PFA) platform.
According to the company,…
The Heart Rhythm Society (HRS) and its new advocacy arm, Heart Rhythm Advocates (HRA),…
Boston Scientific has received an expanded approval from the U.S. Food and Drug Administration (FDA) for its Farapulse Pulsed Field Ablation (PFA) System. More U.S. heart patients are now eligible to be treated…
Two different medical device companies focused on pulsed field ablation (PFA) technology have kicked off July by announcing oversubscribed funding rounds.
Kardium ‘energized’ after raising $250M
Kardium, the…
The number of Americans dying from heart attacks, and ischemic heart disease in general, has decreased dramatically in the last five decades. When it comes to heart failure, atrial fibrillation and a few other complex heart issues, however,…
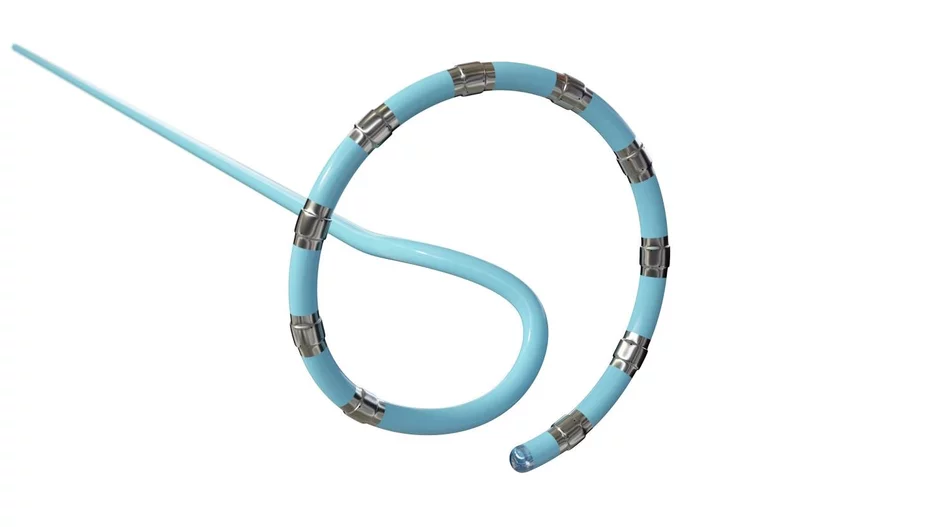
Atraverse Medical, a San Diego-based medtech startup, has raised $29 million in financing to help ramp up the commercialization of its Hotwire radiofrequency (RF) guidewire.
The Hotwire RF…
A bad outcome for Johnson & Johnson MedTech just got significantly worse.
A California judge has entered his judgment in Innovative Health’s lawsuit against Johnson & Johnson’s Biosense Webster division, which now goes by the…
Medicare patients treated with surgical ablation for preexisting atrial fibrillation (AFib) during isolated coronary artery bypass grafting (CABG) procedures are associated with improved survival, according to a new analysis published in The…
Patients with prevalent or incident atrial fibrillation (AFib) after undergoing a successful transcatheter aortic valve replacement (TAVR) procedure…
Johnson & Johnson MedTech has officially launched a new 2D intracardiac echocardiography (ICE) catheter to be used during cardiac ablation procedures.
The…
Innovative Health has been awarded $147 million in damages after the company sued Johnson & Johnson’s Biosense Webster…
Cooling the oblique sinus of the heart may be a safe, effective way to quickly terminate atrial fibrillation (AFib) that develops during a cardiac surgery, according to a new first-in-human study published in Heart Rhythm O2…
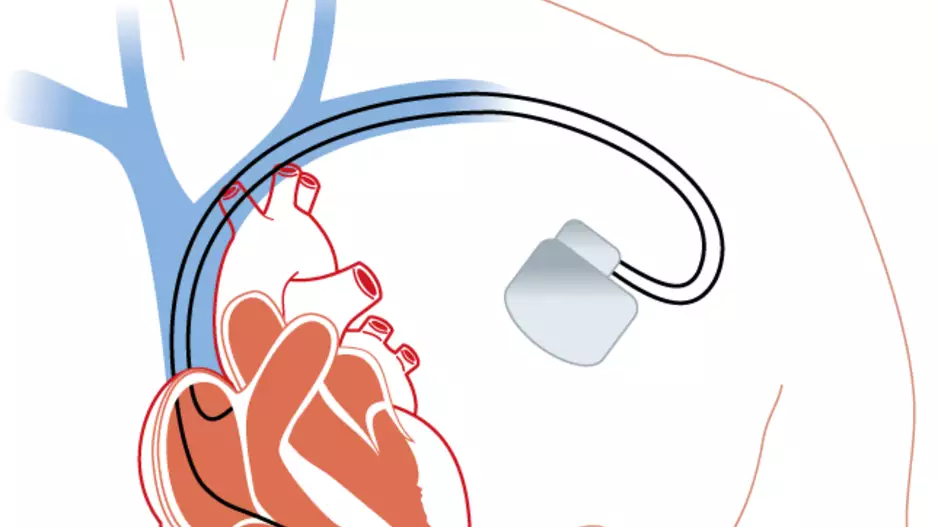
Using a leadless pacemaker to perform leadless left bundle branch area pacing (LBBAP) is both safe and effective, according to new first-in-human data published in Heart Rhythm.[1]
Cardiologists have started turning to leadless…
Artificial intelligence (AI) can help cardiologists anticipate when …
Heart failure patients who use too much cannabis face a much higher risk of experiencing a heart attack or other significant cardiovascular complications, according to new data presented at…