More Articles
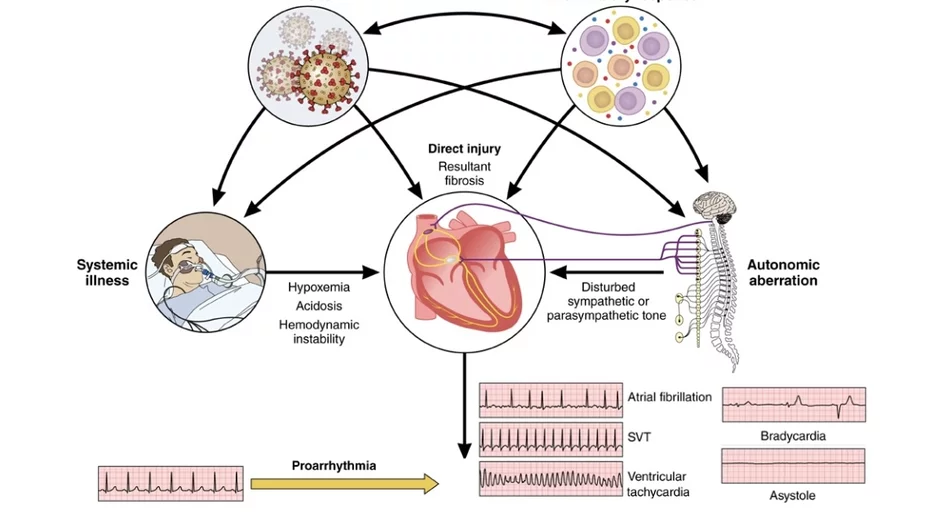
Cardiac arrhythmias are common in patients with COVID-19 infections and during recovery, which prompted a new scientific statement from the…
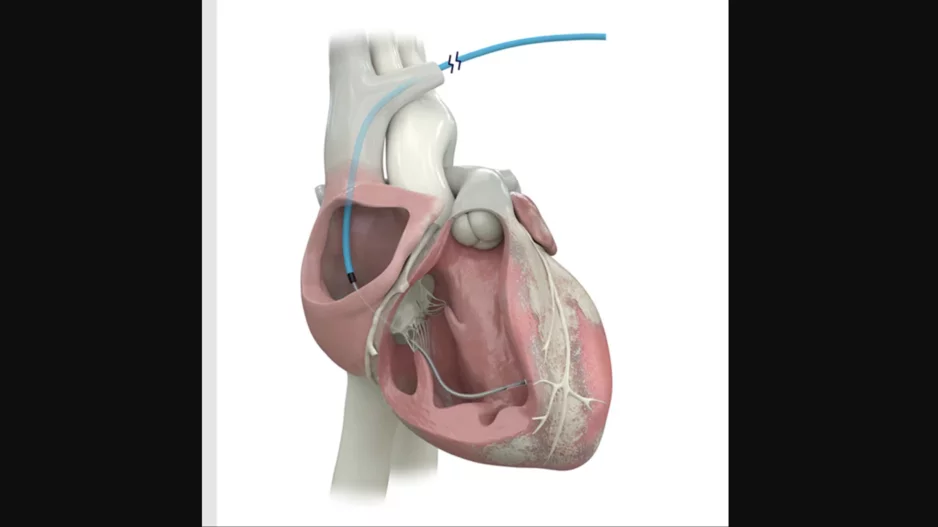
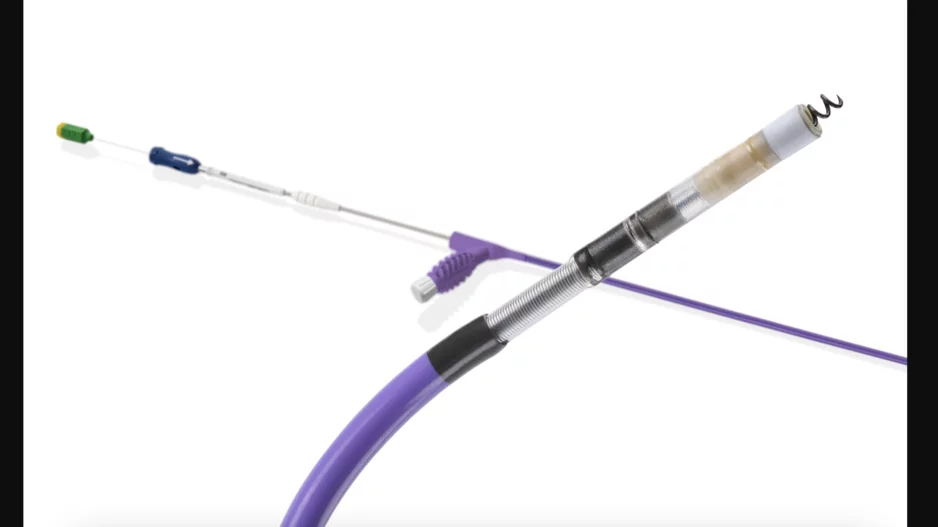
Cardiologists have performed what they believe to be the world’s first substernal lead extraction, sharing their experience in JACC: Case Reports.[1]
The care team, working out of the Regional University Hospital Center of Tours in…
Medtronic’s OmniaSecure defibrillation lead, already associated with positive early outcomes, is projected to deliver long-term durability, according to a new analysis published in HeartRhythm.[1]
The device, which Medtronic…
Patient care in the United States has never been more advanced than it is today, with artificial intelligence and other emerging technologies evolving at a remarkable…
The Heart Rhythm Society (HRS) has shared a new statement emphasizing the importance of atrial fibrillation (AFib) ablation procedures being performed by trained electrophysiologists (EPs).
These…
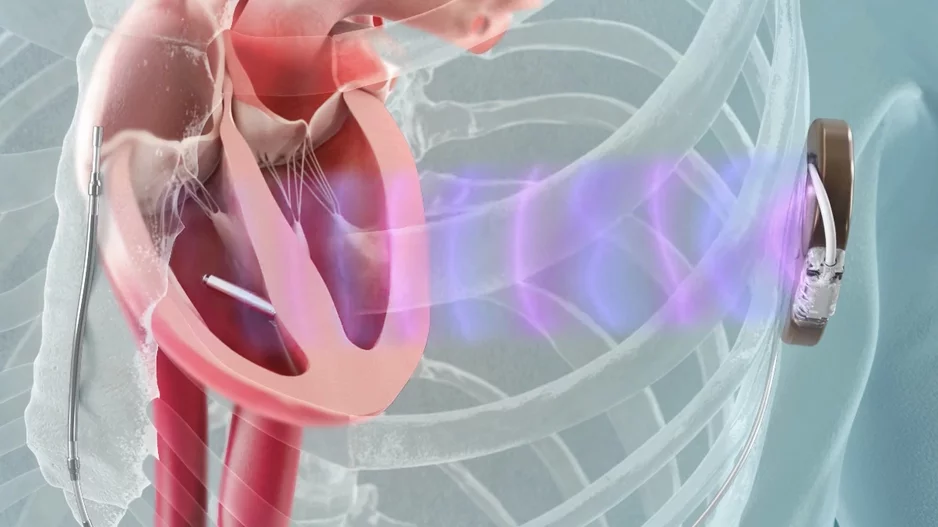
Focal pulsed field ablation (PFA) is a safe, effective treatment option for premature ventricular contractions (PVC), according to new data published in Circulation: Arrhythmia and Electrophysiology.[1]
“Limited evidence on PFA-…
Heart tissue samples that spent 30 days at the International Space Station (ISS) showed low gravity conditions in space weakened the tissues and disrupted their normal rhythmic beats when compared to earth-bound samples from the same source. The…
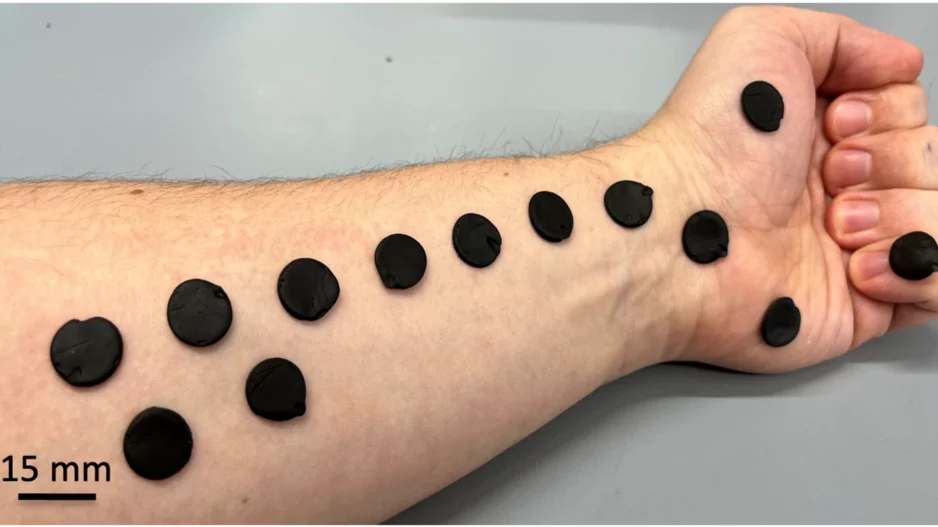
Researchers have found that homemade play-putty can effectively capture electrocardiogram (ECG) signals at a level comparable to commercially…
Left bundle branch area pacing (LBBAP) is associated with better long-term outcomes than traditional right ventricular pacing (RVP) when patients require a permanent pacemaker after…
Boston Scientific has received U.S. Food and Drug Administration (FDA) approval for its Ingevity+ pacing leads to be used for left bundle branch area pacing (LBBAP) when connected to a single- or dual-chamber…
Two U.S. healthcare technology companies have announced a significant transaction that could shake up the country’s electrophysiology market.
Merit Medical Systems, a Utah-based medical device…
Biotronik has received U.S. Food and Drug Administration (FDA) approval to make its Solia S lead and Selectra 3D catheter available for a new indication: left bundle branch area pacing (LBBAP).
LBBAP…
Performing low-voltage-area (LVA) ablation after pulmonary vein isolation (PVI) when treating persistent atrial fibrillation (AFib) does not appear to improve outcomes, according to a new analysis presented at…
The first modular, leadless cardiac rhythm management (CRM) system composed of a leadless pacemaker and a subcutaneous, leadless implantable cardioverter defibrillator (ICD) was linked to positive early results at…
PaceMate, a Florida-based company focused on remote monitoring and patient management technologies, has acquired Medtronic’s…
The U.S. Food and Drug Administration (FDA) has announced that Defibtech, a Nihon Kohden company, is recalling its RMU-2000 ARM XR Chest Compression Device due to significant safety concerns. This is a Class I…
Left atrial appendage occlusion (LAAO) with the Watchman FLX device from Boston Scientific is associated with positive outcomes and limited adverse events after one year, according to new findings published in Circulation: Cardiovascular…
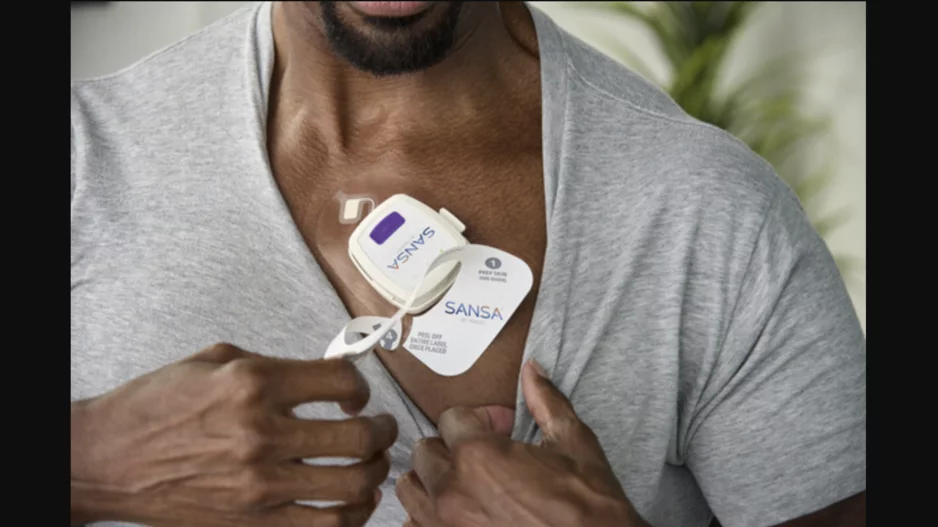
Huxley Medical, an Atlanta-based medical device company, has secured U.S. Food and Drug Administration (FDA) clearance for its new Sansa device, a chest-worn patch…
Treating mitral regurgitation with transcatheter mitral edge-to-edge repair (TEER) using the MitraClip device is associated with a low risk of…
How can cardiologists limit ischemic strokes in patients with atrial fibrillation (AFib) following a successful transcatheter aortic valve replacement (…

![Cardiologists have performed what they believe to be the world’s first substernal lead extraction, sharing their experience in JACC: Case Reports.[1]The device being extracted, Medtronic’s Aurora EV-ICD, received U.S. Food and Drug Administration (FDA) approval in October 2023.](/sites/default/files/styles/media_image/public/2024-10/screenshot_2024-10-11_at_11.56.29_am.png.webp?h=6be7584a&itok=Po6DYlmC)
![Focal pulsed field ablation (PFA) is a safe, effective treatment option for premature ventricular contractions (PVC), according to new data published in Circulation: Arrhythmia and Electrophysiology.[1]](/sites/default/files/styles/media_image/public/2024-09/screenshot_2024-09-25_at_1.12.29_pm.png.webp?h=6be7584a&itok=okd2xvmf)





![Left atrial appendage occlusion (LAAO) with the Watchman FLX device from Boston Scientific is associated with positive outcomes and limited adverse events after one year, according to new findings published in Circulation: Cardiovascular Interventions.[1] Many prior Watchman FLX studies, including PINNACLE FLX, had focused on the device’s performance in a controlled setting. The study’s authors hoped to gain a better understanding of its real-world impact by reviewing registry data from more than 97,000 U.S](/sites/default/files/styles/media_image/public/2024-08/screenshot_2024-08-12_at_11.35.13_am.png.webp?h=6be7584a&itok=djouoZ9Q)
![Treating mitral regurgitation with transcatheter mitral edge-to-edge repair (TEER) using the MitraClip device is associated with a low risk of cerebrovascular accidents (CVAs) such as stroke and transient ischemic attack (TIA), according to new data published in The American Journal of Cardiology.[1]](/sites/default/files/styles/media_image/public/2024-08/small-tech-big-impact-mitraclip-960x430.jpg.webp?h=658ac4ee&itok=0Rn0kdHz)

