More Articles
Cannabis use is on the rise throughout the United States, but it is not as harmless as some people may believe. In fact, according to a new in-depth analysis in Nature Reviews Cardiology, regular cannabis use increases a person’s risk of…
Aortic valve (AV) calcium scores may help cardiologists anticipate when patients face an increased risk of permanent pacemaker implantation (PPMI) after …
Medtronic’s heart rhythm management portfolio, including its insertable cardiac monitors (ICMs) and pulsed field ablation (PFA) systems, have had a significant footprint at AF Symposium 2025, a three-day…
There has been an explosion of interest the past few years in left bundle branch area pacing (LBBAP) as an emerging technique to improve ventricular pacing support by providing more physiologic activation of cardiac tissue than conventional…
The U.S. Food and Drug Administration (FDA) has announced that Philips is recalling the software associated with its Mobile Cardiac Outpatient Telemetry (MCOT) devices after certain high-risk electrocardiogram (…
Johnson & Johnson MedTech has received CE mark approval for its Dual Energy ThermoCool SmartTouch SF Catheter for treating patients with cardiac arrhythmias.
The device help users switch between radiofrequency and pulsed field…
Johnson & Johnson MedTech has temporarily paused the use of its Varipulse pulsed field ablation (PFA) system within the United States after it was linked to four “neurovascular events” in patients who underwent treatment.
The…
A care team in Illinois has performed the first heart procedure of its kind on a two-year-old patient diagnosed with Brugada syndrome.
The pediatric patient suffered a sudden cardiac arrest at home and was revived when his parents…
The American College of Cardiology (ACC) recently published a new expert consensus document on practical approaches for arrhythmia monitoring after stroke. The guidance offers clinicians tailored strategies to improve…
The U.S. Food and Drug Administration (FDA) has announced that Boston Scientific is recalling the catheters associated with its…
Cardiologists have made a bit of history in Prague, performing what are believed to be the world’s first leadless left bundle branch area pacing (LBBAP) procedures. The procedures were all completed using Abbott’s investigational Aveir conduction…
Merit Medical, a Utah-based medical device company with more than 7,000 employees, has announced the resignation of its president, Joseph C. Wright. The move is effective Jan. 3, 2025…
The U.S. Food and Drug Administration (FDA) and Boston Scientific today sent an urgent alert to patients and healthcare providers about the…
While the technology and techniques associated with transcatheter aortic valve replacement (TAVR) have advanced over the years, treating patients who…
The diagnosis and management of clinically suspected acute myocarditis is an important topic for clinicians from a variety of backgrounds, including cardiologists, primary care physicians, emergency physicians and rheumatologists.
According…
The U.S. Senate has unanimously passed the HEARTS Act, HR.6829, a bipartisan bill designed to improve cardiomyopathy education and awareness while getting more…
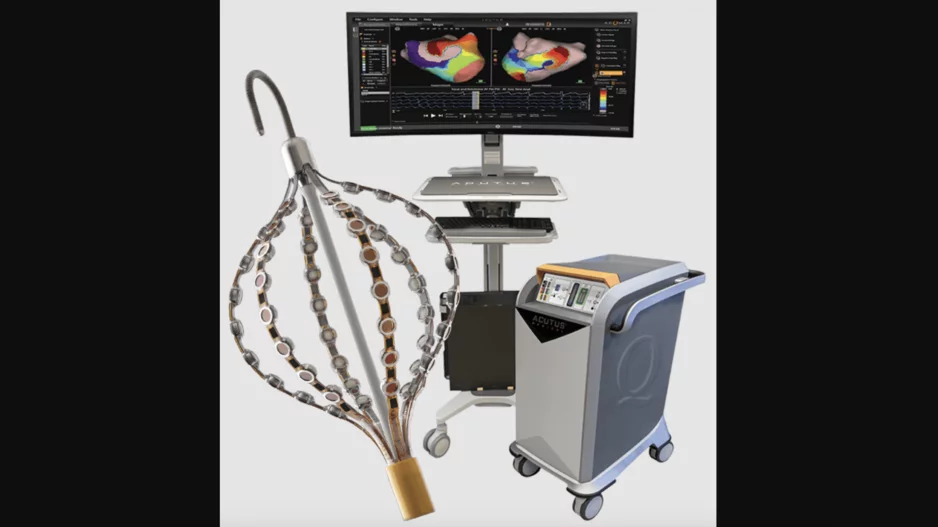
Acutus Medical, the California-based healthcare technology company focused on electrophysiology devices, has announced…
AOP Health, an Austrian pharmaceutical company, has received U.S. Food and Drug Administration (FDA) approval for landiolol to be used in a critical care setting for the treatment of supraventricular tachycardia…




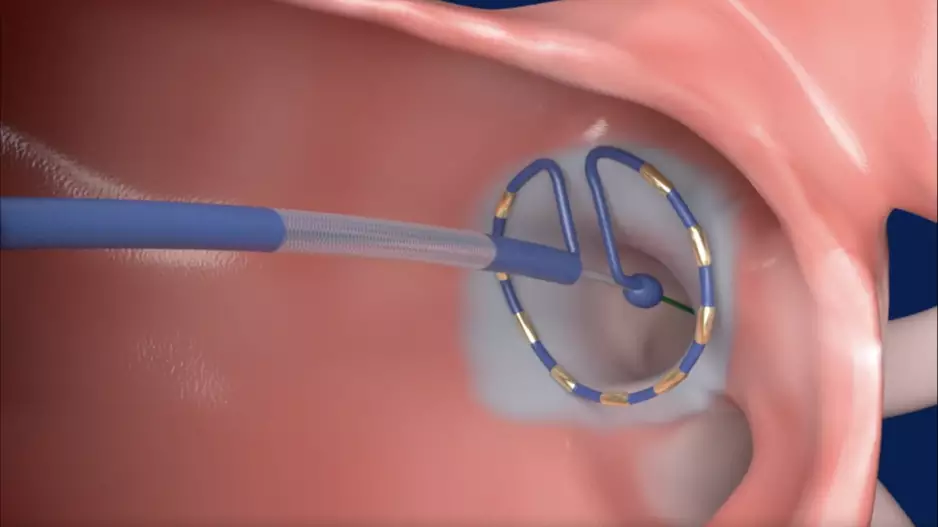













![Left atrial appendage occlusion (LAAO) with the Watchman FLX device from Boston Scientific is associated with positive outcomes and limited adverse events after one year, according to new findings published in Circulation: Cardiovascular Interventions.[1] Many prior Watchman FLX studies, including PINNACLE FLX, had focused on the device’s performance in a controlled setting. The study’s authors hoped to gain a better understanding of its real-world impact by reviewing registry data from more than 97,000 U.S](/sites/default/files/styles/media_image/public/2024-08/screenshot_2024-08-12_at_11.35.13_am.png.webp?h=6be7584a&itok=djouoZ9Q)