More Articles
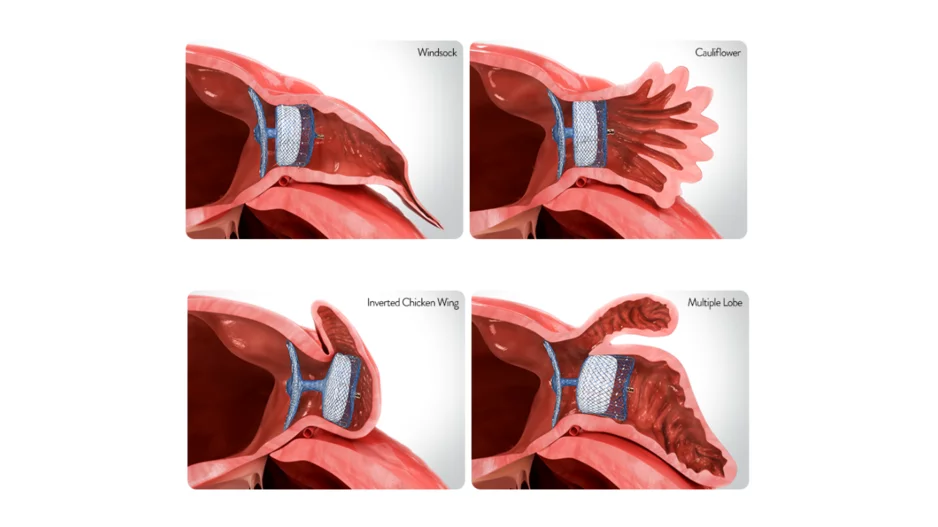
Advancements in radiofrequency (RF) catheter ablation technology have been incremental over the past 30 years in efforts to improve safety, procedural efficiency and patient outcomes. While some newer technologies have gained a lot of attention…
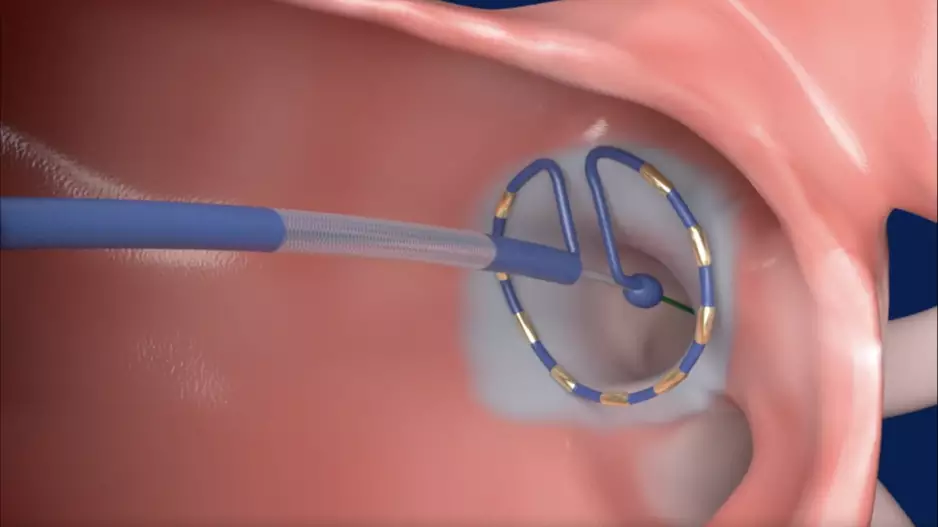
The Amplatzer Amulet Left Atrial Appendage Occluder (LAAO) from Abbott is safe and effective a full five years after treatment, according to new data published in the Journal of the American College of Cardiology.[1]
The study’s…
Advanced artificial intelligence (AI) algorithms could help cardiologists identify atrial fibrillation (AFib) patients on direct oral anticoagulants (DOACs) who face…
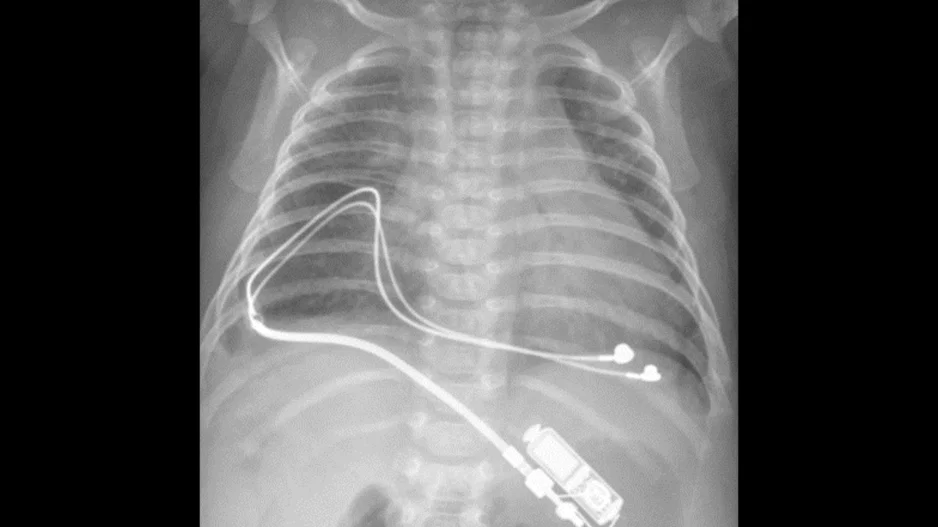
Small pacemakers built specifically for infants are both safe and effective, according to a new analysis published in Circulation: Arrhythmia and Electrophysiology.[1]
“There are two challenges with placing a pacemaker in a small…
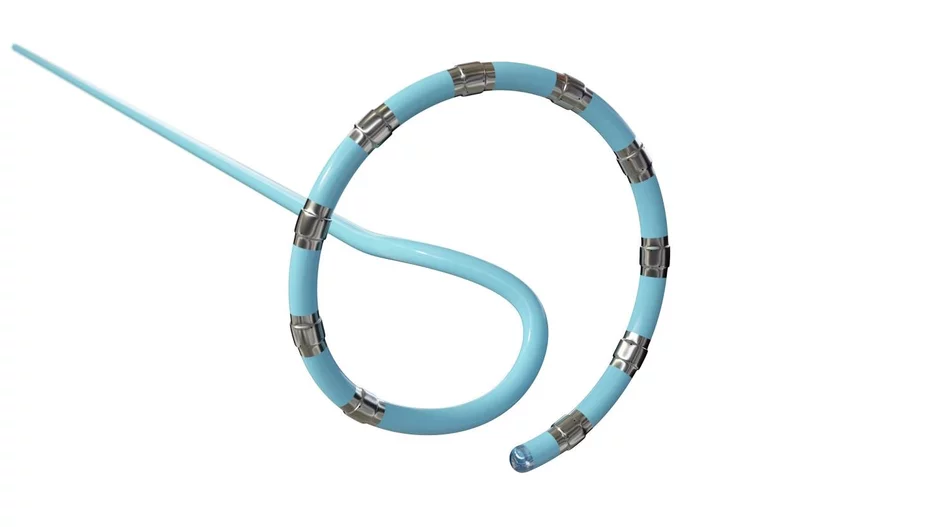
The U.S. Food and Drug Administration (FDA) has announced a new recall for the ablation catheters associated with Johnson & Johnson MedTech’s Varipulse pulsed field ablation (PFA) system. No devices need to…
Kestra Medical Technologies, a Washington-based medtech company known for its wearable heart monitors and defibrillators, is going public on the Nasdaq Global Select Market under the ticker symbol KMTS.
The company intends to sell 10…
According to the U.S. Food and Drug Administration (FDA), safety issues with certain Boston Scientific pacemakers are significant enough that the situation has been categorized as a a Class I recall. This means…
Johnson & Johnson MedTech has resumed the U.S. rollout of its Varipulse pulsed field ablation (PFA) system after previously putting cases on hold to review reports of patient complications. The Varipulse PFA system works as intended, the…
Researchers have developed a new artificial intelligence (AI) algorithm that can identify atrial fibrillation (AFib) patients who may benefit from left atrial…
Novartis has agreed to acquire Boston-based Anthos Therapeutics for up to $3.1 billion. The deal includes an upfront payment of $925 million and $2.15 billion in additional payments based on certain regulatory and commercial milestones.
…
Adults who regularly floss their teeth may be significantly decreasing their risk of stroke or heart rhythm issues, according to new findings to be presented at the…
A majority of Americans use a device to monitor their heart health, according to a new survey commissioned by The Ohio State University Wexner Medical Center. However, just 25% of those users take that extra step and share their data with a…
Royal Philips has agreed to sell its emergency care business to Bridgefield Capital, a private equity firm based in Washington, D.C. Financial terms of this deal were not shared with the public.
Philips’ emergency care business includes its…
Cannabis use is on the rise throughout the United States, but it is not as harmless as some people may believe. In fact, according to a new in-depth analysis in Nature Reviews Cardiology, regular cannabis use increases a person’s risk of…
Aortic valve (AV) calcium scores may help cardiologists anticipate when patients face an increased risk of permanent pacemaker implantation (PPMI) after …
Medtronic’s heart rhythm management portfolio, including its insertable cardiac monitors (ICMs) and pulsed field ablation (PFA) systems, have had a significant footprint at AF Symposium 2025, a three-day…
There has been an explosion of interest the past few years in left bundle branch area pacing (LBBAP) as an emerging technique to improve ventricular pacing support by providing more physiologic activation of cardiac tissue than conventional…
The U.S. Food and Drug Administration (FDA) has announced that Philips is recalling the software associated with its Mobile Cardiac Outpatient Telemetry (MCOT) devices after certain high-risk electrocardiogram (…
Johnson & Johnson MedTech has received CE mark approval for its Dual Energy ThermoCool SmartTouch SF Catheter for treating patients with cardiac arrhythmias.
The device help users switch between radiofrequency and pulsed field…