More Articles
Element Science, a California-based medtech company, has gained U.S. Food and Drug Administration (FDA) approval for its Jewel Patch Wearable Cardioverter…
Pulsed field ablation (PFA) is growing more and more popular among electrophysiologists all over the world. It was also a…
Drinking champagne may be associated with significant cardiovascular benefits, according to a new study published in the Canadian Journal of Cardiology.[1]
The study identified dozens of lifestyle changes that may help lower a…
Treatment with finerenone can help patients with cardiovascular-kidney-metabolic (CKM) syndrome reduce their risk of developing new-onset atrial fibrillation (AFib) or atrial flutter (AFL), according to a new pooled analysis published in the …
Pulsed field ablation (PFA) technologies were the center of attention at Heart Rhythm 2025, the Heart Rhythm Society’s annual meeting in San Diego.
PFA is an ablation…
Medtronic has received U.S. Food and Drug Administration (FDA) approval for its…
Artificial intelligence (AI) can improve atrial fibrillation (AFib) outcomes by providing real-time feedback during cardiac ablation procedures, according to new data…
The Heart Rhythm Society (HRS) and American College of Cardiology have collaborated on a new scientific statement focused on the practice of same-day discharge (SDD)…
Field Medical, a California-based pulsed field ablation (PFA) company founded in 2022, is set to receive significant attention at Heart Rhythm 2025, the Heart Rhythm Society’s…
The world is in the throes of an atrial fibrillation (AFib) pandemic, one that could potentially get worse in the years ahead due to population growth, economic hardships and rigid resource limitations.
The most effective way to…
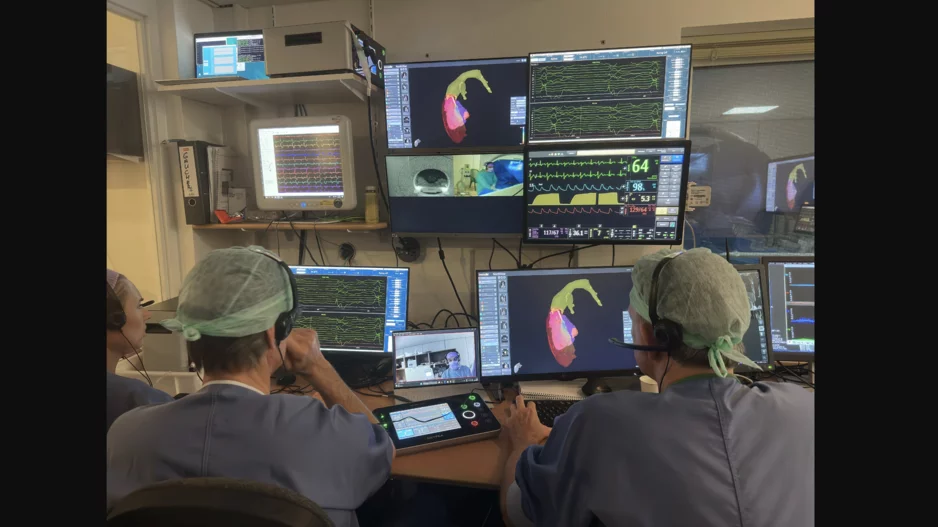
Cardiologists and imaging specialists at Amsterdam University Medical Center (AUMC) have performed the world’s first ventricular ablation procedure guided by real-time MRI images.
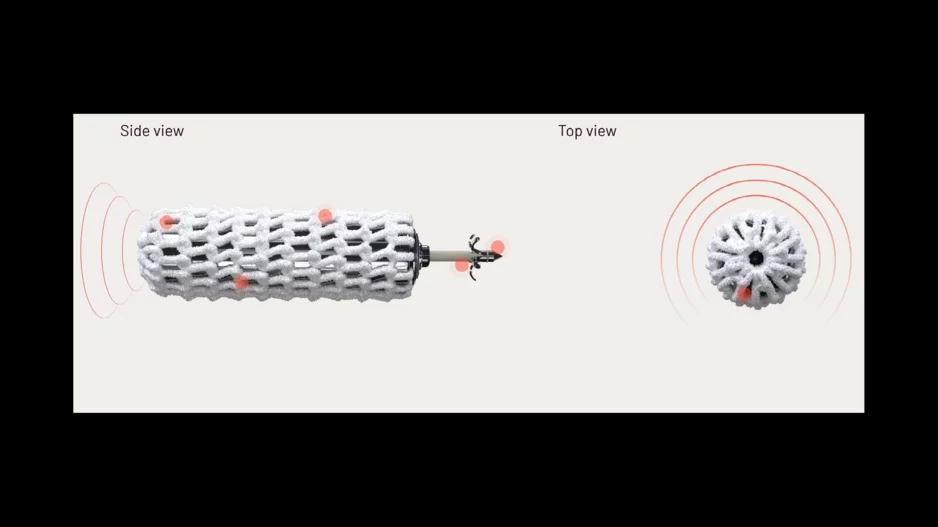
LUMA Vision, a medical device company based out of Ireland and Germany, has received U.S. Food and Drug Administration (FDA) clearance for its new catheter-based visualization technology designed to help…
EBR Systems, a California-based medtech company, has gained U.S. Food and Drug Administration (FDA) approval for its innovative wireless pacing device for heart failure, the…
Just a week after taking office as the new American College of Cardiology (ACC) President, Christopher Kramer, MD, FACC, was in Washington, D.C.,…
CardioVia, an Israeli MedTech company, has gained U.S. Food and Drug Administration (FDA) clearance for its ViaOne technology designed to help clinicians reach the…
Outpatient cardiology practices that participate in an accountable care organization (ACO) as part of the Medicare Shared Savings Program (MSSP) do not experience immediate improvements in the quality of care they provide, according to new…
The Heart Rhythm Society (HRS) has established is own legislative advocacy group Heart Rhythm Advocates (HRA), which has begun operations in Washington,…
The U.S. Food and Drug Administration (FDA) has informed Milestone Pharmaceuticals that it is not approving etripamil, the company’s new nasal spray for heart rhythm issues, at this time.
Etripamil…
Abbott has received CE mark approval in Europe for its new Volt Pulsed Field Ablation (PFA) System…
Exposure to scatter radiation and orthopedic issues related to years of wearing lead aprons during long EP procedures has led electrophysiologists to seek out new ways to reduce the need for angiographic X-ray. The Texas Cardiac Arrhythmia…










![A majority of medical devices involved in Class I recalls were never required by the U.S. Food and Drug Administration (FDA) to undergo premarket or postmarket clinical testing, according to new research published in Annals of Internal Medicine.[1]](/sites/default/files/styles/media_image/public/2024-09/istock-1209664264.jpg.webp?h=97a06f33&itok=FbSiOdtK)