More Articles
Viz.ai has received a de novo approval from the U.S. Food and Drug…
The U.S. Food and Drug Administration (FDA) has issued a new draft guidance designed to help applicants add clinically relevant information about QTc interval prolongation to the labeling of non-antiarrhythmic…
Can fasting instructions before transcatheter aortic valve replacement (TAVR), ablation and other cardiac procedures be relaxed? A team of researchers…
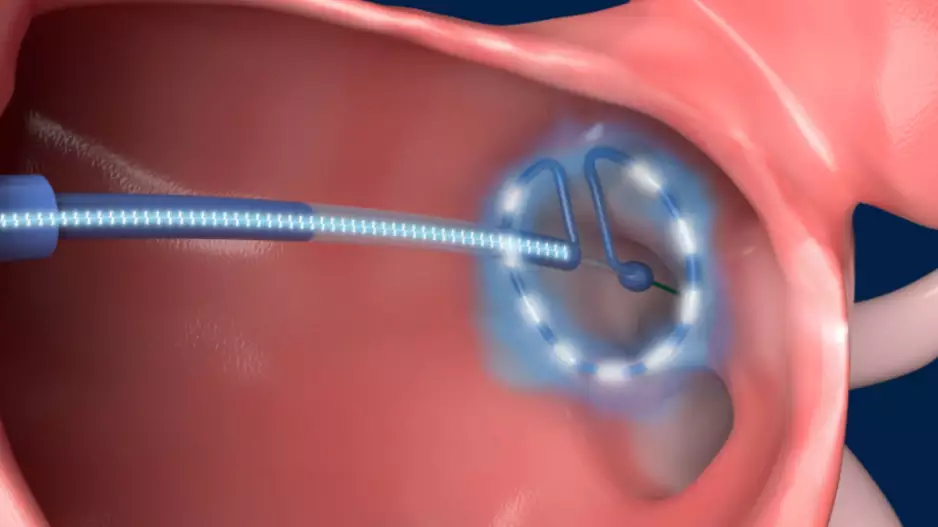
The U.S. Food and Drug Administration (FDA) has approved Boston Scientific’s POLARx Cryoablation System for the treatment of patients with atrial fibrillation (AFib).
The system includes the expandable…
NFL player feeling thankful after AFib diagnosis: ‘I think it’s just the good Lord looking after me’
Tyler Shatley, a veteran offensive lineman with the Jacksonville Jaguars, was diagnosed with atrial fibrillation (AFib) on Aug. 2 after experiencing symptoms for the last few years. Shatley discussed the diagnosis with the media on Aug. 6,…
Biosense Webster, a Johnson & Johnson company, has received U.S. Food and Drug Administration (FDA) approval for multiple devices from its cardiac ablation portfolio to be used as part of a zero-fluoroscopy…
Expanding Medicaid eligibility is associated with significant improvements in care for low-income patients with coronary artery disease, heart failure,…
The U.S. Food and Drug Administration (FDA) has announced that GE Healthcare is recalling more than 7,000 TruSignal SpO2 sensors due to issues with the devices malfunctioning and sending a reduced amount of…
Patients who develop atrial fibrillation (AFib) for the first time after transcatheter aortic valve replacement (TAVR) face a heightened risk of all-…
PaceMate, the Florida-based healthcare technology company focused on remote cardiac monitoring, has received a new “strategic growth investment” from Lead Edge Capital, a well-known tech investor that has previously worked with such groups as…
Targeting immune cells that play a key role in the development of atrial fibrillation (AFib) could give cardiologists a new way to treat the disease, according to a new analysis in Science.[1] The study’s authors said this approach could…
The U.S. Food and Drug Administration (FDA) has announced that Medtronic is recalling nearly 350,000 implantable cardiac devices due to continued issues with their ability to deliver high voltage therapy when…
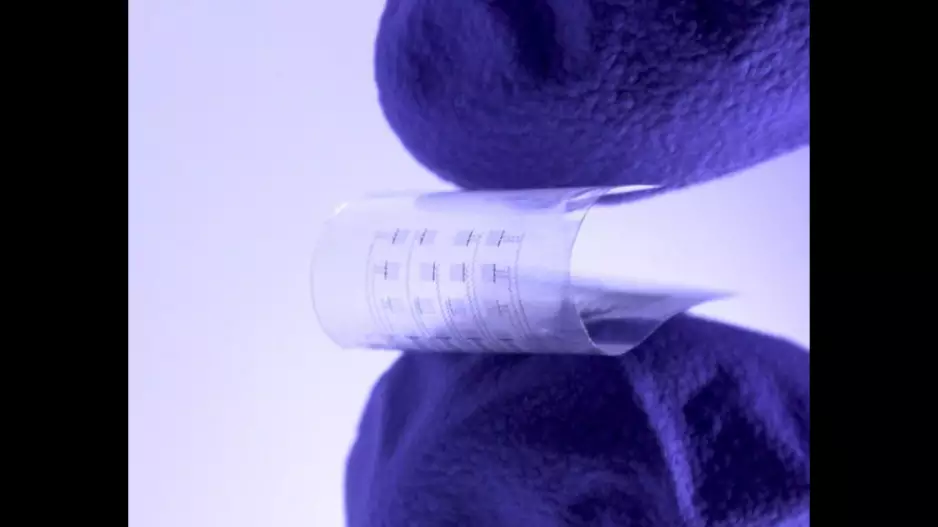
Researchers have developed new heart monitors that dissolve inside the body when they are no longer needed, sharing their work in Science Advances.[1]
The new-look devices are soft, flexible, transparent and roughly the size of a…
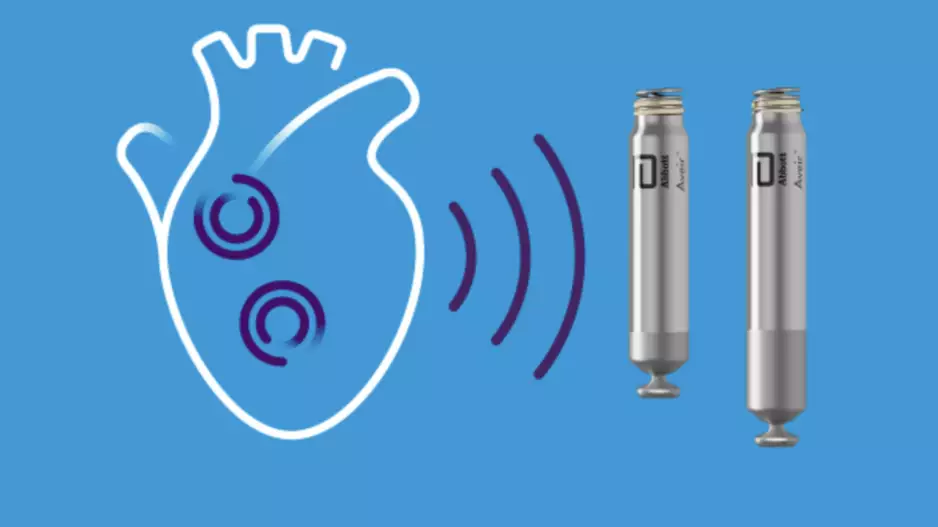
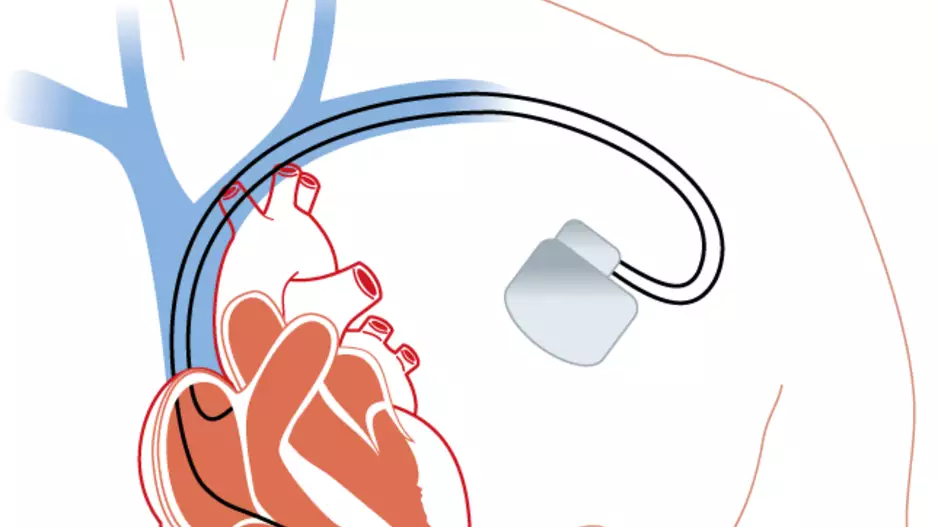
The U.S. Food and Drug Administration (FDA) has approved Abbott’s Aveir dual chamber (DR) leadless pacemaker system, the world’s very first dual-chamber leadless pacing solution for treating patients with…
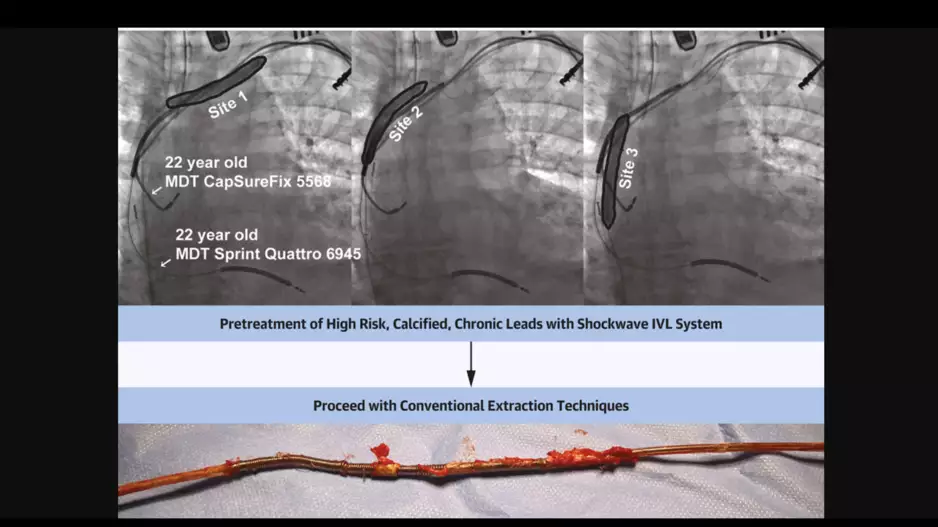
Intravascular lithotripsy (IVL) can provide significant value during transvenous lead extraction (TLE) procedures by identifying dense calcifications, according to new research published in JACC: Clinical Electrophysiology.[1]
“…
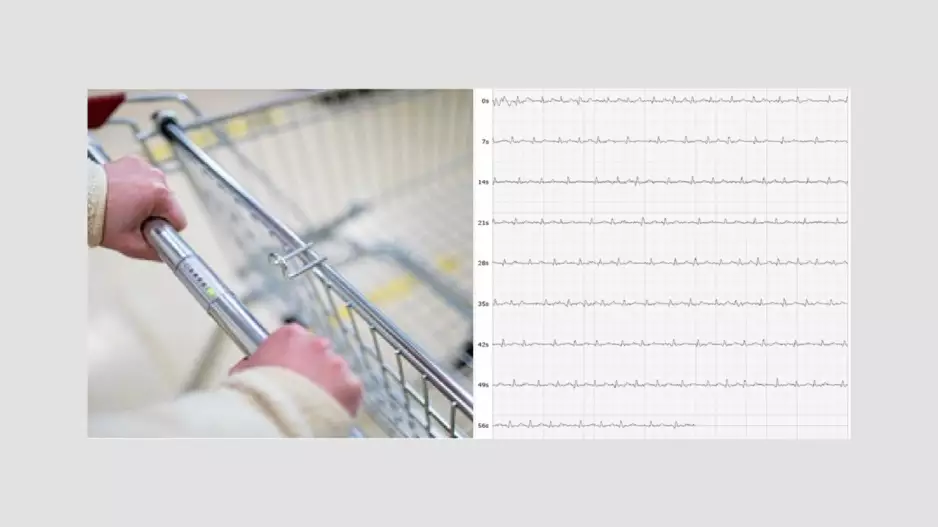
Adding electrocardiogram (ECG) sensors to shopping carts can helps identify patients who may have atrial fibrillation (AFib) or other…
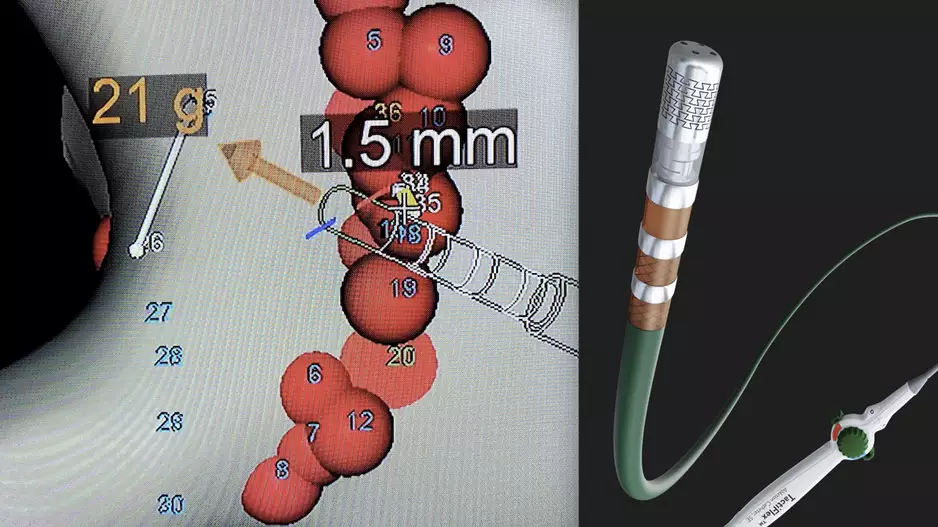
The U.S. Food and Drug Administration (FDA) recently cleared Abbott's TactiFlex Ablation Catheter, Sensor Enabled, which is the the world's first ablation catheter with a flexible tip and contact force technology. The radiofrequency (RF) catheter…
‘Notably high’ rates of PTSD, depression and anxiety seen in patients with implantable heart devices
Post-traumatic stress disorder (PTSD), depression and anxiety are all much more common among patients who receive an implantable cardioverter defibrillator (ICD) than the general population, according to new research published in EP Europace…
One of the biggest technology trends in cardiac electrophysiology is the the development of pulsed field ablation (PFA), a non-thermal ablation treatment for patients with atrial fibrillation (AF). The Heart…
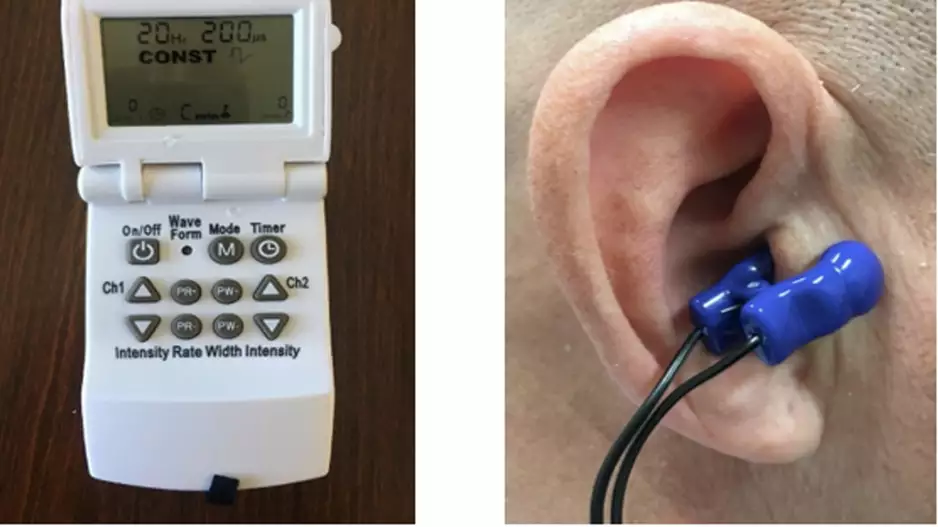
A patient-administered therapy ear clip device effectively uses nerve stimulation to treat postural tachycardia syndrome (POTS), according to a late-breaking clinical trial at Heart Rhythm 2023 [1]. This may offer a new treatment option for…
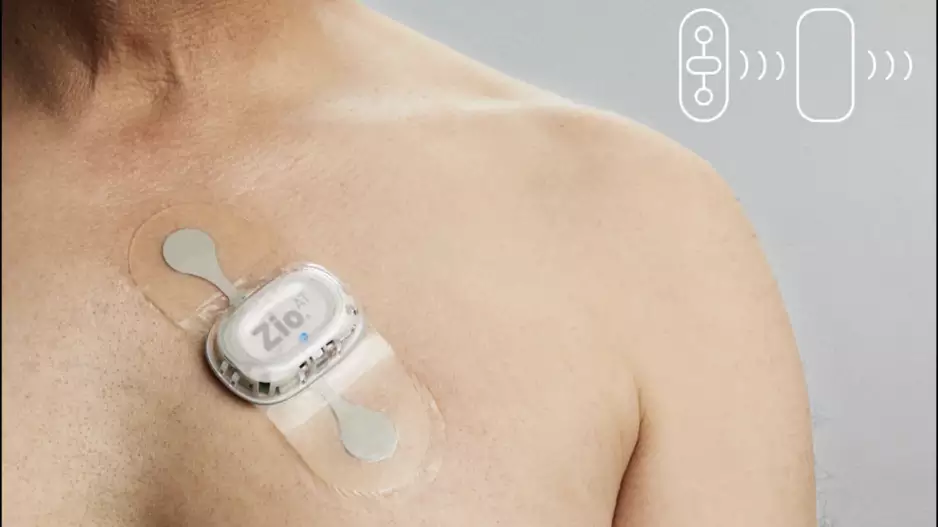
The U.S. Food and Drug Administration (FDA) sent a warning letter to iRhythm Technologies detailing several issues related to the company’s Zio AT mobile cardiac telemetry device. The letter was sent on May 25…