More Articles
Conformal Medical, a New Hampshire-based medical device company founded in 2016, has completed a Series D funding round worth $35 million. The new investments come as the company continues developing its…
An artificial intelligence (AI) algorithm that analyses electrocardiograms…
Results from a pivotal clinical trial presented as a late-breaker at Heart Rhythm 2023 indicate that a new leadless…
Two late-breaking clinical studies at Heart Rhythm 2023 highlight the success of left bundle branch area pacing (…
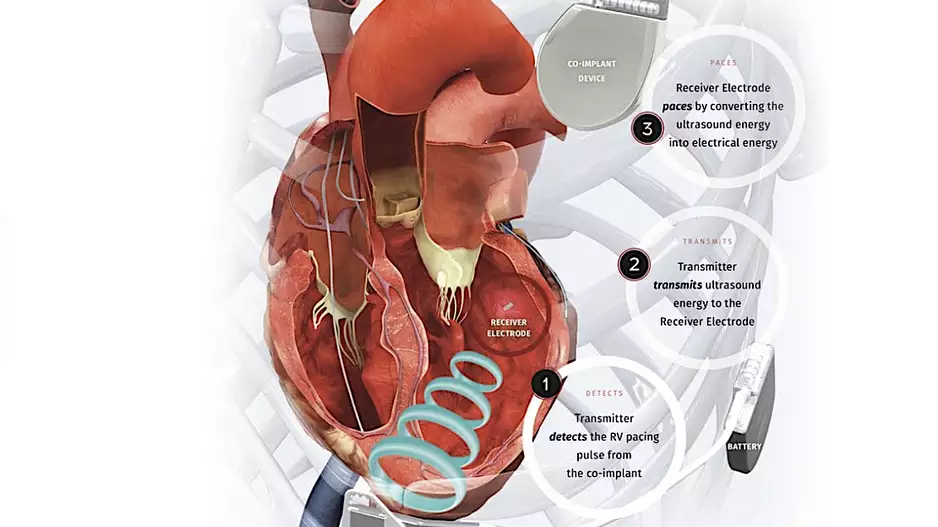
Two veteran cardiologists from Cleveland Clinic have performed the first heart procedure of its kind, successfully implanting a new dual cardiac device in a patient presenting with heart failure symptoms.
The procedure was part of the…
Abbott has received U.S. Food and Drug Administration (FDA) approval for its Assert-IQ insertable cardiac monitor (ICM). The device, designed for the long-term monitoring of arrhythmias, uses Bluetooth…
Asundexian, a new investigational drug from Bayer, is now one step closer to being approved by the U.S. Food and Drug Administration (FDA) to treat atrial fibrillation (AFib).
The company announced…
Ablation is an effective, beneficial treatment option for young adults with atrial fibrillation (AFib), according to new five-year data published in Circulation: Arrhythmia and Electrophysiology.[1] The study’s authors paid close…
The Heart Rhythm Society (HRS) has released the full list of late-breaking electrophysiology (EP) studies…
Samsung Electronics has gained U.S. Food and Drug Administration (FDA) clearance for a new Irregular Heart Rhythm Notification (IHRN) feature that is part of its…
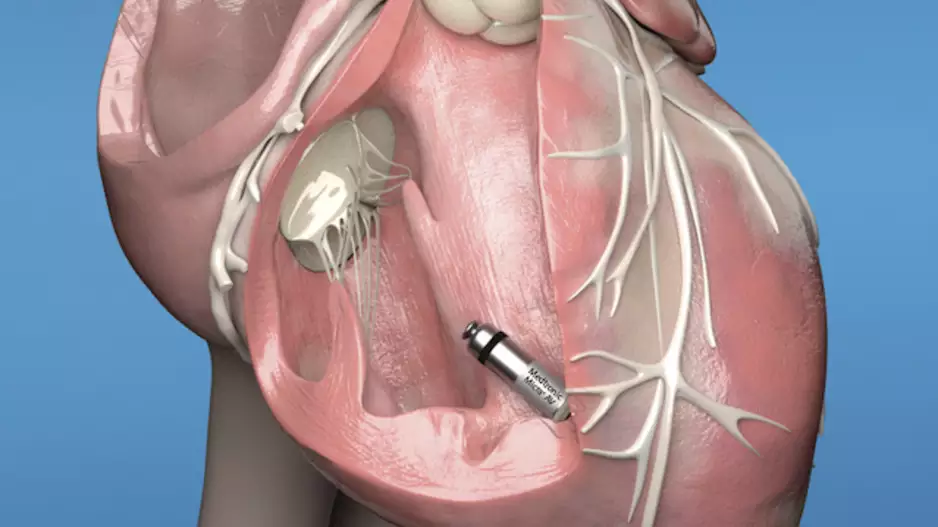
Medtronic has received U.S. Food and Drug Administration approval for its next-generation Micra leadless pacemakers, the…