More Articles
The U.S. Food and Drug Administration (FDA) has granted the first pediatric indication for an implantable cardiac monitor to Medtronic. The…
More than 5% of patients hospitalized with COVID-19 go on to develop new-onset atrial fibrillation (AFib), according to new research published in Circulation:…
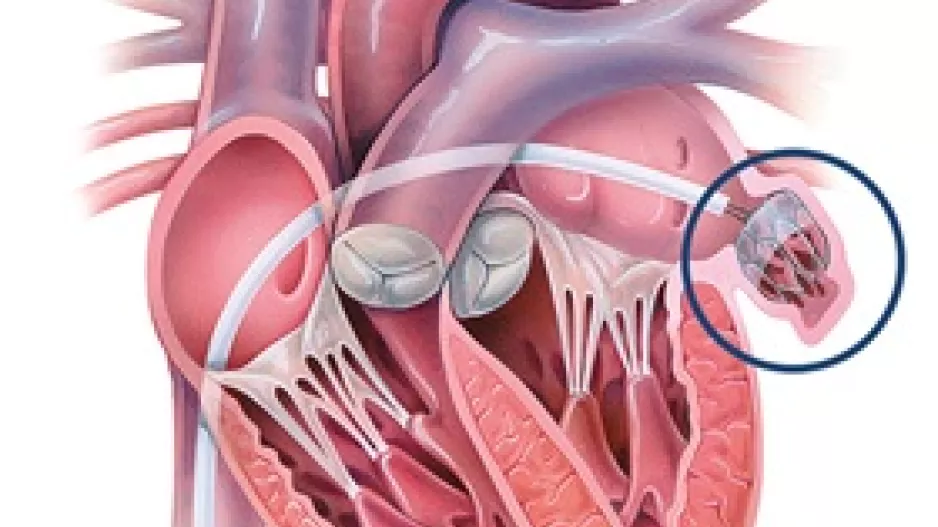
Moderate-to-severe and severe mitral regurgitation (MR) are significantly associated with a heightened risk of death among older patients with heart failure with preserved…
The U.S. Food and Drug Administration (FDA) has approved a clinical trial to evaluate the safety and efficacy of a new type of ablation catheter for patients with ventricular tachycardia (VT) resistant to conventional anti-arrhythmic drugs or…
Boston Scientific has received approval from the U.S. Food and Drug Administration (FDA) to expand the labeling for its Watchman FLX left atrial appendage closure (LAAC) device. The updated instructions will now…
Cardiac implantable electronic devices (CIEDs) such as pacemakers and implantable defibrillators are associated with a higher infection risk than many cardiologists realize. One study, in fact, found that as many as…
It has long been believed that men face a higher risk of developing atrial fibrillation (AFib) than women. A new study published in JAMA Cardiology, however, flips the script on that very notion, suggesting that the risk is actually much…
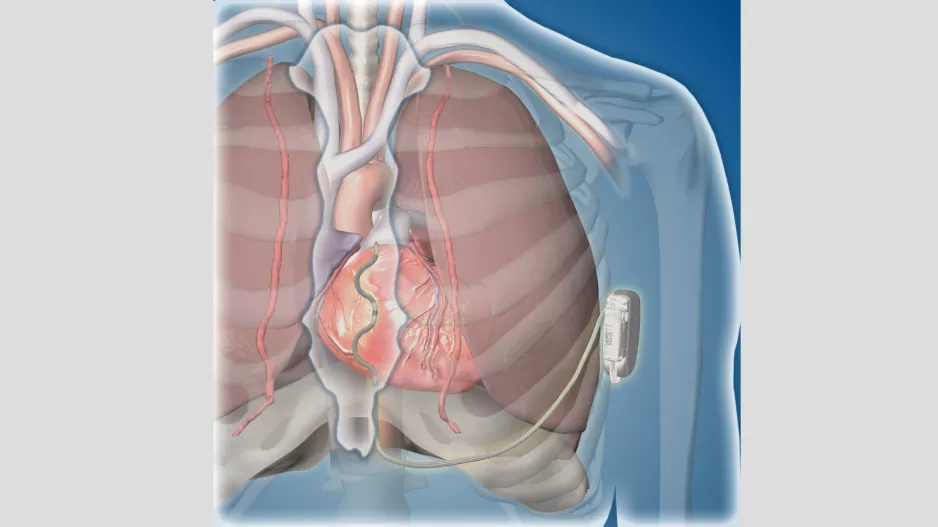
Medtronic shared some good news with attendees at ESC Congress 2022 in Barcelona, the annual meeting of the European Society of Cardiology, noting that its Extravascular Implantable Cardioverter…
ESC Congress 2022, the annual meeting of the European Society of Cardiology, was jam-packed with eye-opening new research from many of the leading voices in cardiovascular and vascular medicine.
…
Treating elderly atrial fibrillation (AFib) patients—even those who are traditionally ineligible for direct oral anticoagulants (DOACs)—with a very low dose of edoxaban is associated with improved outcomes, according to new research published in…
Thinking has changed: Cardiac implantable electronic device (CIED) infections are a bigger deal than we thought.
The latest research supports a new approach to who and how we treat, says veteran cardiologist…
Patients prescribed cannabis for chronic pain may face a heightened risk of experiencing heart rhythm issues, according to new research to be presented at…
The U.S. Food and Drug Administration (FDA) has announced that Medtronic is recalling 87,709 implantable cardiac devices due to the risk of a malfunction that could result in serious injury or death. …
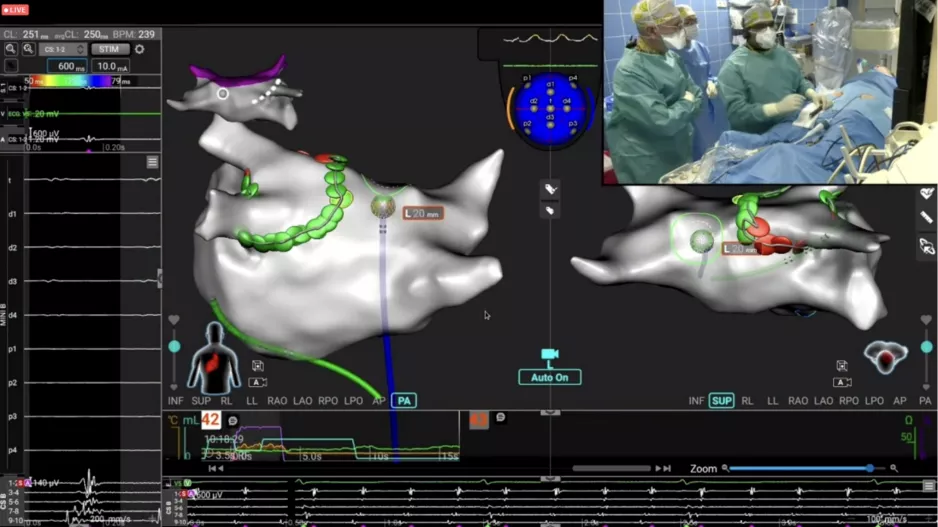
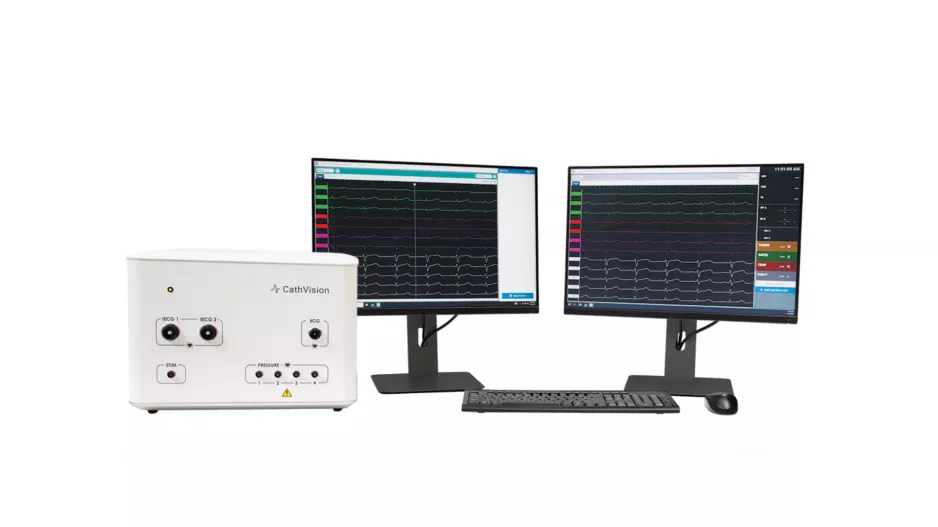
CathVision, a Denmark-based healthcare technology company focused on the electrophysiology (EP) lab, has closed a new financing round worth $7.2 million. The funding…
Monitoring patient symptoms is an essential part of diagnosing and treating cardiovascular disease (CVD). A new scientific statement from the American Heart Association (AHA), published in Circulation…
Atrial cardiomyopathy is associated with an increased risk of dementia among older adults, according to new research published in the Journal of the American Heart Association.[1]
“Stroke and atrial fibrillation (AFib) are two…
In a massive simulation exercise, researchers recently found that using wrist-worn wearable devices to screen people for atrial fibrillation (AFib) may be more cost-effective than traditional modalities such as pulse palpation and 12-…
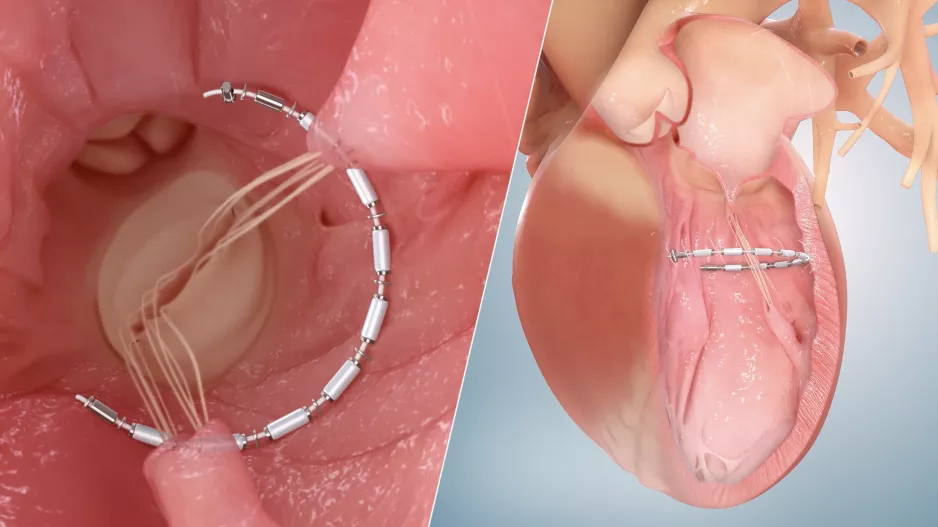
The risk of in-hospital mortality may be significantly higher among transcatheter edge-to-edge repair (TEER) patients who present with concomitant…
There is not agreement among some of the top structural heart experts who perform left atrial appendage occlusion (LAAO) if cardiac computed tomography (CT…