Cardiology Associations
This page includes news coverage of cardiology societies and associations. Follow these links for specific cardiology society news pages: American College of Cardiology (ACC), American Heart Association (AHA), American Society of Echocardiography (ASE), American Society Nuclear Cardiology (ASNC), European Society of Cardiology (ESC), Heart Rhythm Society (HRS), Society for Cardiovascular Angiography Interventions (SCAI), Society of Cardiovascular Computed Tomography (SCCT), Transcatheter Cardiovascular Therapeutics (TCT), and Vascular Interventional Advances (VIVA).
Displaying 169 - 176 of 1417

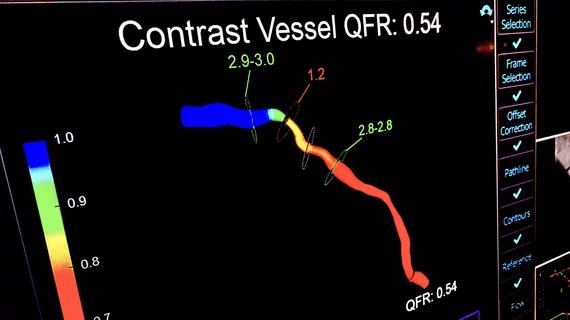


![The use of intravascular lithotripsy (IVL) during percutaneous coronary intervention (PCI) is still safe and effective when patients present with calcified nodules (CNs), according to new long-term data published in EuroIntervention.[1] Researchers compared outcomes from patients with and without CNs, highlighting key similarities in stent expansion and luminal gain.](/sites/default/files/styles/top_stories/public/2024-12/screenshot_2024-12-02_at_11.07.21_am.png.webp?itok=YDi6SyF_)