Pharmaceutics
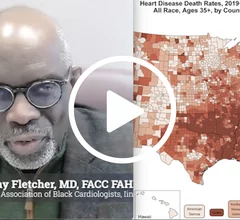
This page contains key pharmaceutical news on drug recalls, FDA clearance, safety communications and research. In cardiology, key pharmaceutic agents include antiplatelet therapies, anticoagulants, hypertension drugs, and drugs for heart failure and arrhythmias.
Displaying 1 - 8 of 422