More Articles
Patients who develop atrial fibrillation (AFib) for the first time after transcatheter aortic valve replacement (TAVR) face a heightened…
The U.S. Food and Drug Administration (FDA) has announced that Medtronic is recalling nearly 350,000 implantable cardiac devices due to continued issues with their ability to deliver high voltage…
Researchers have developed a new artificial intelligence (AI) model capable of evaluating cardiac function and identifying signs of valvular heart…
Following a ketogenic diet could potentially benefit certain heart failure patients, according to a new commentary published in JACC: Heart Failure.[1] However, additional research on this topic is still needed. …
Managing heart failure (HF) symptoms after patients undergo percutaneous coronary intervention (PCI) can help reduce their long-term risk of a major…
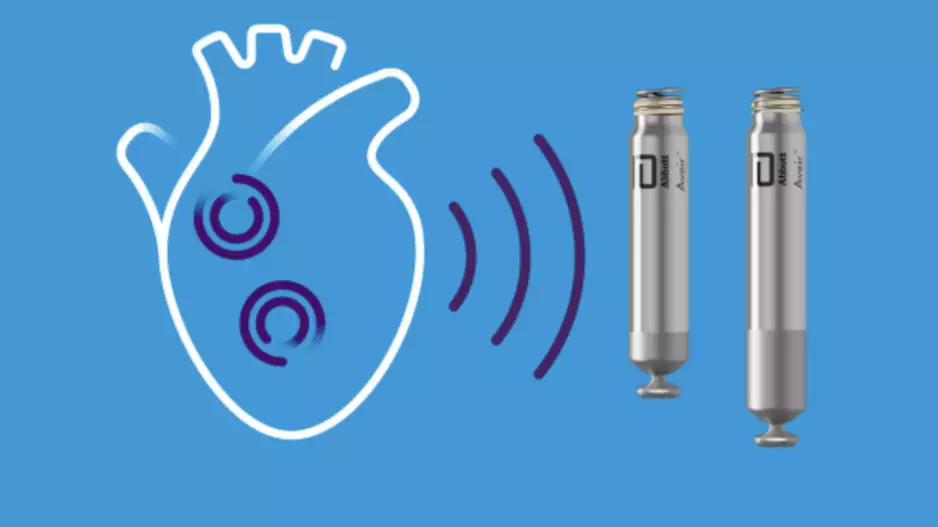
The U.S. Food and Drug Administration (FDA) has approved Abbott’s Aveir dual chamber (DR) leadless pacemaker system, the world’s very first dual-chamber leadless pacing solution for treating patients…
A team of interventional cardiologists has made a bit of history, using a new transcatheter device to treat tricuspid regurgitation (TR) in a high-risk heart failure patient who with a previously implanted left ventricular assist…
Cardiologists and other physicians have always believed cardiac transthyretin amyloidosis (ATTR-CM), a progressive heart condition associated with a high…
The U.S. Food and Drug Administration (FDA) has published a new letter highlighting a voluntary recall of certain Getinge/Maquet medical devices used during cardiopulmonary bypass (CPB) and…
The U.S. Food and Drug Administration (FDA) has announced that Abiomed is recalling more than 450 of its Impella heart pumps due to a heightened risk of purge…
Sotagliflozin has been approved by the U.S. Food and Drug Administration (FDA) as a daily treatment to reduce the risk of cardiovascular death, hospitalization for…
Researchers have used advanced artificial intelligence (AI) models to identify five different…
Two veteran cardiologists from Cleveland Clinic have performed the first heart procedure of its kind, successfully implanting a new dual cardiac device in a patient presenting with heart failure symptoms.
…
AstraZeneca has announced that dapagliflozin, which it sells under the name Farxiga, is now approved by the U.S. Food and Drug Administration (FDA) to reduce the risk of cardiovascular death,…
The American Heart Association (AHA) has awarded a total of $15 million to three research teams to study the association between chronic stress and cardiovascular disease (CVD). …
Magenta Medical, an Israel-based healthcare technology company, announced that it has closed a financing round worth $55 million. The funds are expected to support the continued development of its…
CVRx, a medical device company known for its novel Barostim technology that uses neuromodulation to improve the symptoms of patients with heart failure, has shared financial and operational data from the…
A new laser imaging technology may be able to determine which donor hearts are viable for transplant and which will result in poor outcomes without the need for coronary angiography or use of contrast agents that can damage an explanted heart.…
Abbott has received two new clearances from the U.S. Food and Drug Administration (FDA) for its…
The American College of Cardiology (ACC) has issued two new guidance documents focused on the treatment and management of …

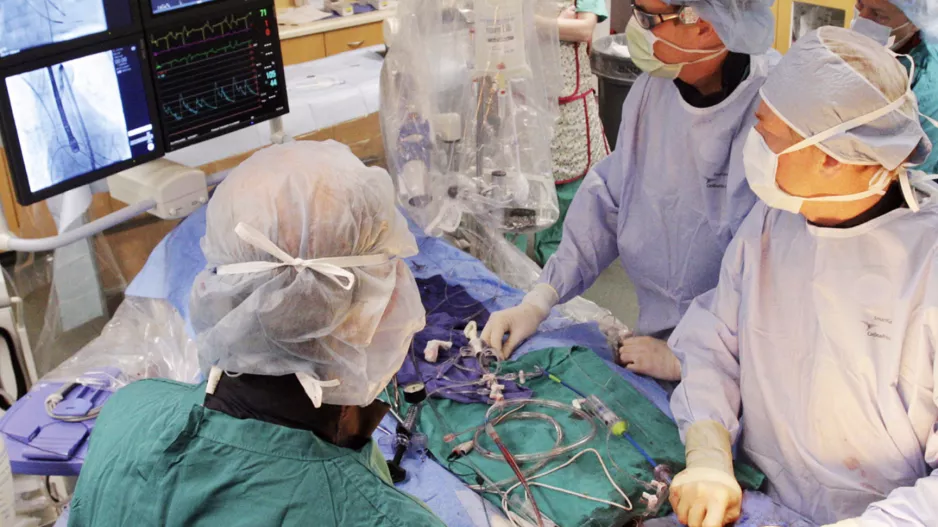

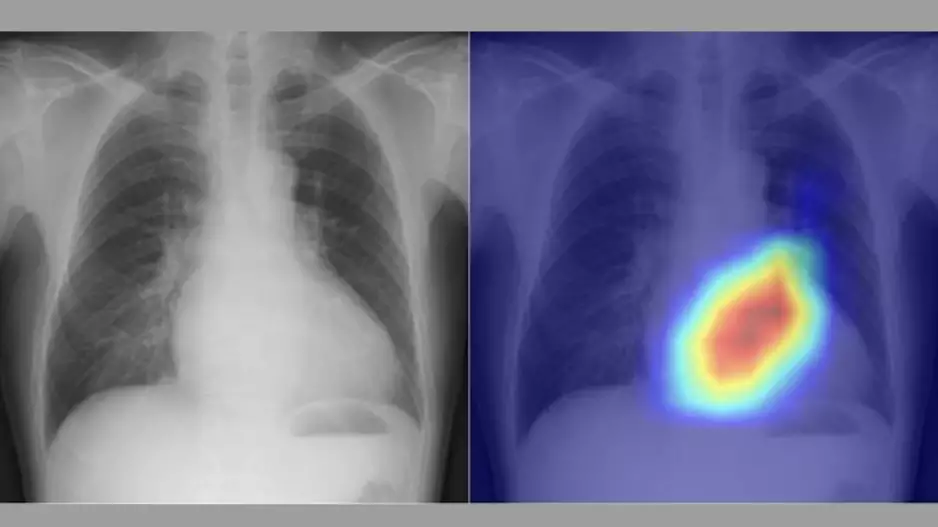


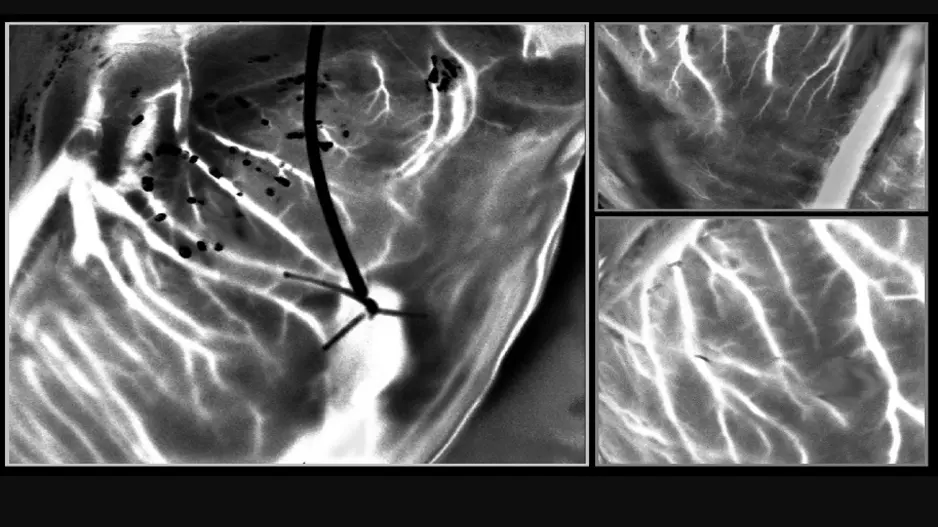
![Large peridevice leaks after left atrial appendage occlusion (LAAO) are incredibly rare and not associated with a greater risk of adverse outcomes, according to new research published in JACC: Clinical Electrophysiology.[1] Smaller residual links are more common, however, and associated with a risk of thromboembolic and bleeding events.](/sites/default/files/styles/media_image/public/2018-08/olderpatient-doctor.jpg.webp?itok=WkwdpD4_)