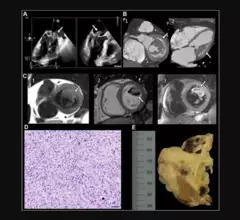
Mitral Valve
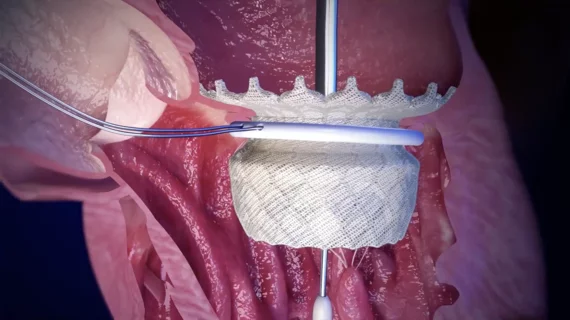
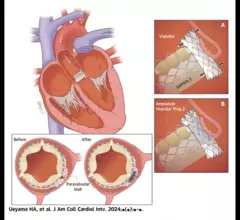
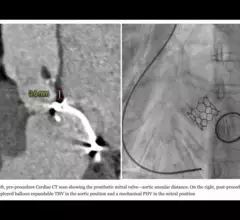
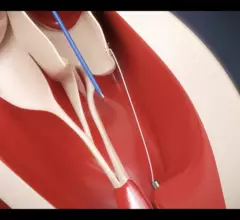
The heart's mitral valve is the site of the most surgical valve repairs and valve replacements. After the resounding success of transcatheter aortic valve replacement (TAVR), which now makes up more than 50% of aortic valve replacements, there is wide expectation transcatheter mitral replacements will follow in the next few year. Currently, the most common transcatheter mitral procedure is transcatheter edge-to-edge (TEER) , using the MitraClip or Pascal clip devices. These devices are also being used for transcatheter tricuspid valve repair (TTVR). Other transcatheter mitral repair systems are in trials for minimally invasive annuloplasty and chordae tendineae repair.