Heart Failure
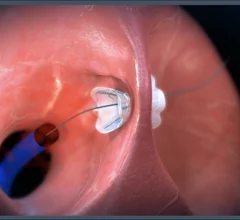
Heart failure occurs when the heart cannot pump as much blood as the body requires. This ineffective pumping can lead to enlargement of the heart as the myocardium works harder pump the same amount of blood. Heart failure may be caused by defects in the myocardium, such as an a heart attack infarct, or due to structural issues such as severe heart valve regurgitation. Heart failure can be divided into HF with preserved ejection fraction (HFpEF), and HF with reduced ejection fraction (HFrEF). The disease is further divided into four New York Heart Association (NYHA) classes. Stage IV heart failure is when the heart is completely failing and requires a heart transplant or hemodynamic support from a left ventricular assist device (LVAD).