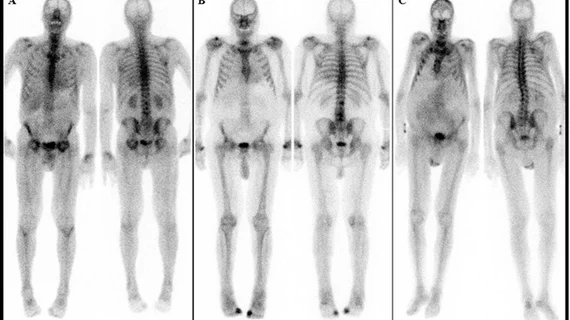
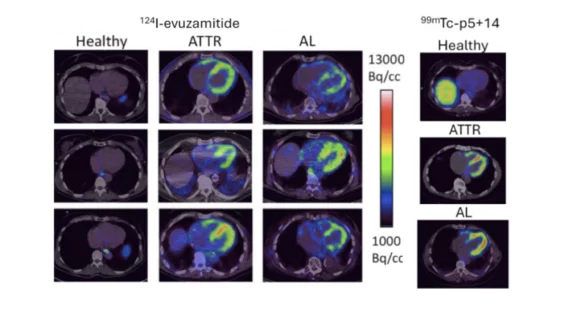
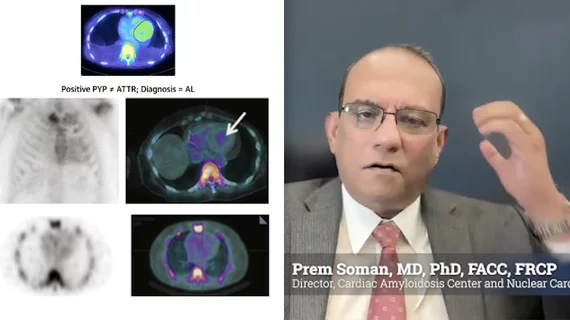
Cardiac Amyloidosis
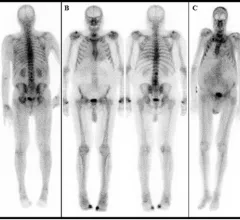
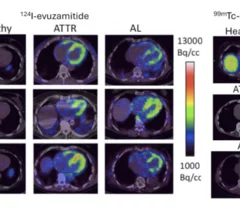
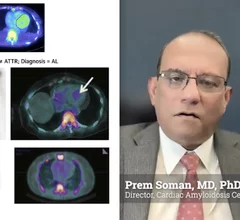
With the first drug treatments for cardiac amyloidosis recently entering the market, there has been an explosion of interest to diagnose and care for these patients. It is considered a rare disease, but many experts now say it is actually just be under diagnosed. The disease is caused by protein misfolding. Normally soluble proteins in the bloodstream become insoluble and deposit abnormally in the tissues and organs throughout the body. There are three main kinds of amyloid that affect the heart, light chain amyloid (AL) and two types of transthyretin amyloid (ATTR or TTR). The first type of ATTR is hereditary, or familial amyloid, and the second is wild type, or age-related TTR amyloid. Nuclear imaging, echocardiography, CT and MRI all play roles in diagnosing amyloid and in determining the subtype, which is required for targeted treatment.