More Articles
The American College of Cardiology (ACC) has launched a new partnership with Amgen and Esperion Therapeutics aimed at improving LDL screening among patients with and without a history of…
LimFlow, a medical device company with locations in California and Paris, has gained approval from the U.S. Food and Drug Administration (FDA) for its LimFlow System…
The number of obesity-related cardiovascular deaths in the United States has tripled in the last two decades, according to new research published in the Journal of the American Heart Association.[1] Black women have been hit…
Postoperative delirium is a common side effect of transcatheter aortic valve replacement (TAVR), according to a new meta-analysis published…
Patients who take statins following an intracerebral hemorrhage—also commonly known as a bleeding stroke—may reduce their risk of an additional stroke, according to new data published in Neurology.[1]
…
Intravascular imaging-guided percutaneous coronary intervention (PCI) is associated with significant benefits compared to angiography-guided PCI, according to a new meta-analysis presented at…
The Texas Heart Institute (THI) in Houston, recently ranked one of the top 20 U.S. hospitals for cardiology and heart…
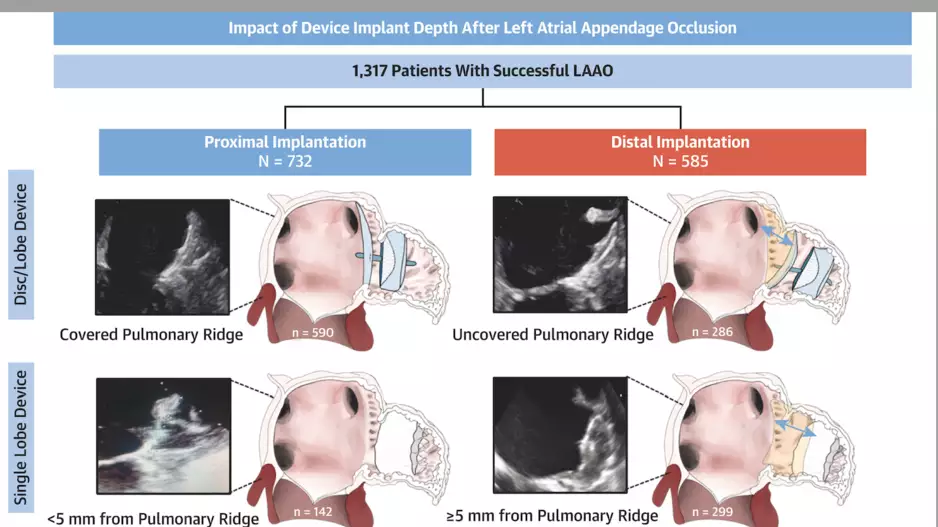
Deeper device implantation during left atrial appendage occlusion (LAAO) procedures increases the patient’s risk of developing a blood clot, according to new research published in JACC: Cardiovascular Interventions.[1]…
The U.S. Food and Drug Administration (FDA) has announced that Abiomed is recalling the labeling for its Impella RP Flex with SmartAssist catheter.
This is a…
The 2022 nationwide shortage of intravenous iodinated contrast media made a clear impact on stroke care in the United…
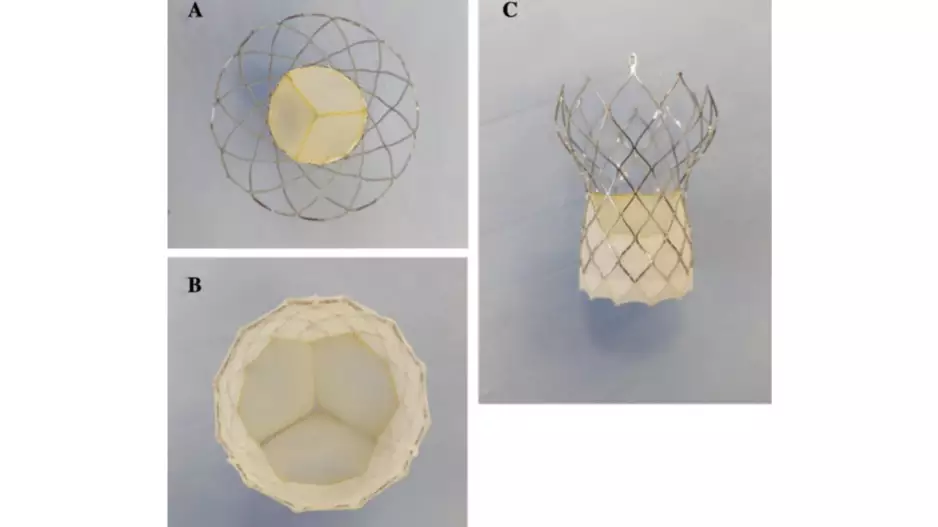
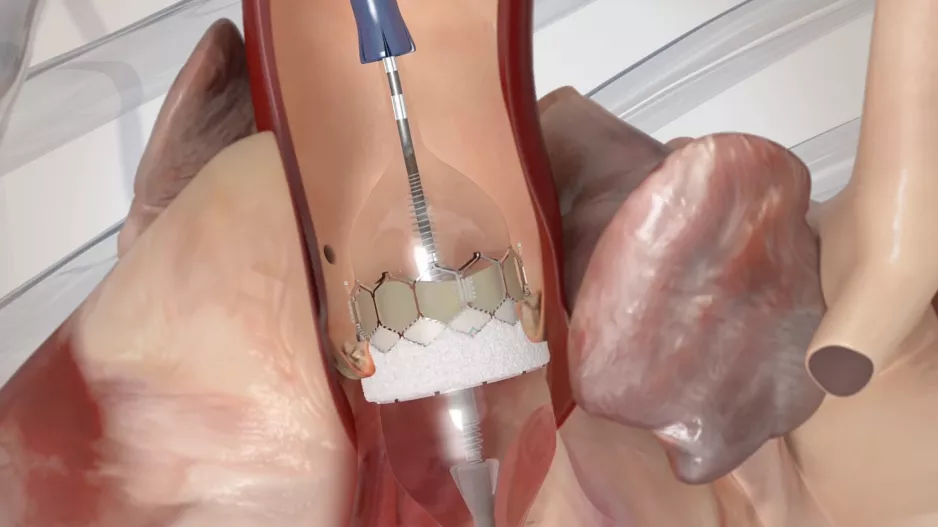
Interventional cardiologists have performed transcatheter aortic valve replacement (TAVR) with a new self-expandable, supra-annular valve…
Weekly doses of semaglutide, a GLP-1 receptor originally developed to treat diabetes, could help approximately 93 million U.S. adults lose weight and reduce their risk of adverse cardiovascular events, according to new research…
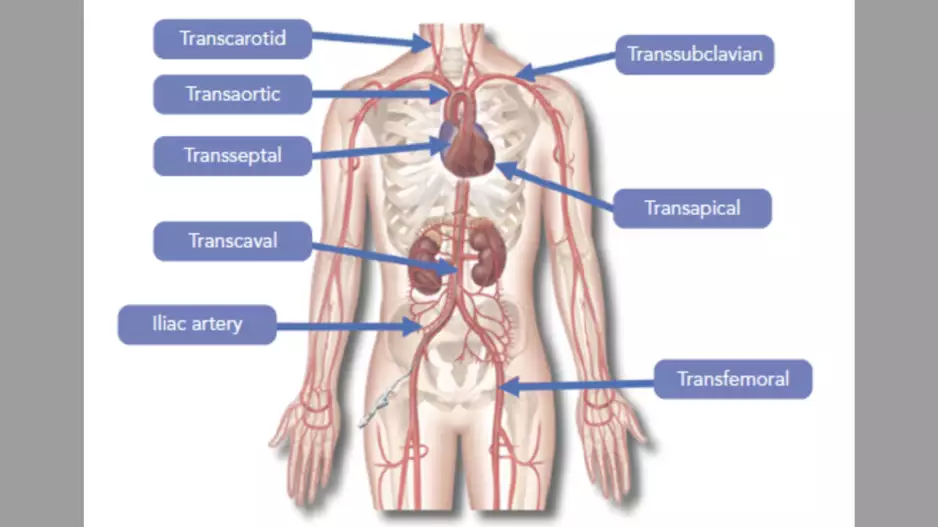
When transfemoral access is not recommended for transcatheter aortic valve replacement (TAVR), how should cardiologists and the rest of the…
Expanding Medicaid eligibility is associated with significant improvements in care for low-income patients with coronary artery disease, heart failure,…
The CardioVascular Coalition (CVC) has shared its concerns about payment cuts included in the…
Researchers from the Icahn School of Medicine at Mount Sinai and Weill Cornell Medicine, both located in New York City, have secured $29.9 million in…
The U.S. Food and Drug Administration (FDA) has updated its stance on using paclitaxel-coated devices to treat peripheral artery disease (PAD), reversing restrictions originally put in place back in…
Managing heart failure (HF) symptoms after patients undergo percutaneous coronary intervention (PCI) can help reduce their long-term risk of a major…
When treating intermediate-risk patients, transcatheter aortic valve replacement (TAVR) with a modern balloon-expandable heart valve and…
The U.S. Food and Drug Administration (FDA) has announced that Arrow International, a subsidiary of Teleflex, is recalling more than 250,000 catheters after receiving reports of device separation and…
Cardiovascular Associates of America (CVAUSA), a Florida-based practice management company operated by Webster Equity Partners, has acquired Bay Area…