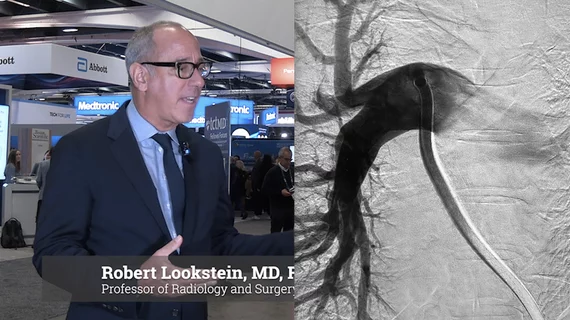
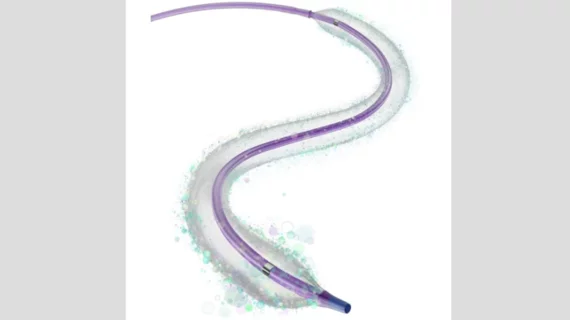
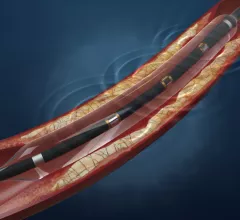
Vascular Interventional Advances (VIVA)
The VIVA Foundation is a not-for-profit organization dedicated to advancing the field of vascular medicine and intervention through education, research, advocacy, and collaboration. It hosts the VIVA (Vascular InterVentional Advances) and The VEINS (Venous Endovascular INterventional Strategies) annual conferences. The VIVA Foundation also presents the Vascular Leaders Forum and collaborates with other industry-leading groups to move the field forward.
Displaying 1 - 8 of 11