Nuclear Medicine
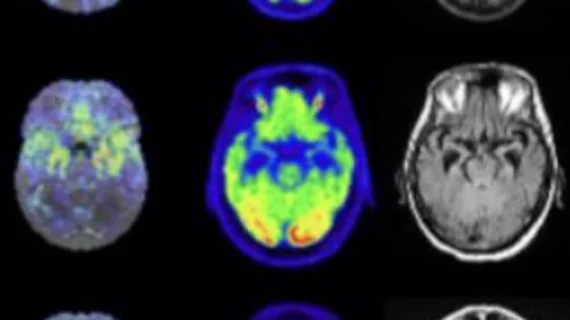
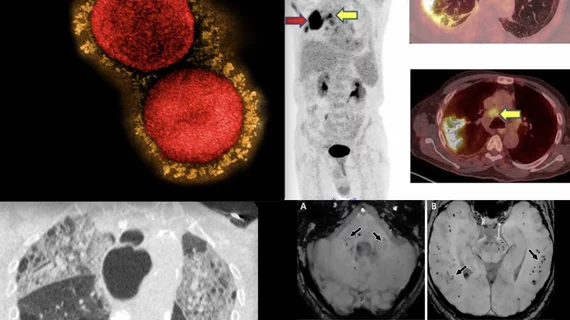
Nuclear medicine (also called molecular imaging) includes positron emission computed tomography (PET) and single photon emission computed tomography (SPECT) imaging. Nuclear imaging is achieved by injecting small amounts of radioactive material (radiopharmaceuticals) into patients before or during their scan. These can use sugars or chemical traits to bond to specific cells. The radioactive material is taken up by cells that consume the sugars. The radiation emitted from inside the body is detected by photon detectors outside the body. Computers take the data to assemble images of the radiation emissions. Nuclear images may appear fuzzy or ghostly rather than the sharper resolution from MRI and CT. But, it provides metabolic information at a cellular level, showing if there are defects in the function of the heart, areas of very high metabolic activity associated with cancer cells, or areas of inflammation, data not available from other modalities. These noninvasive imaging exams are used to diagnose cancer, heart disease, Alzheimer’s and Parkinson’s disease, bone disorders and other disorders.