Vascular & Endovascular
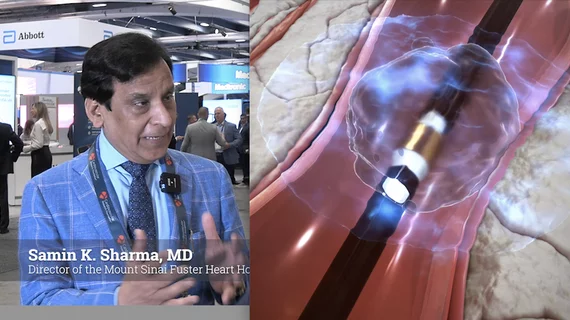
This channel includes news on non-coronary vascular disease and therapies. These include peripheral artery disease (PAD), abdominal and thoracic aortic aneurysm (AAA and TAA), aortic dissection, pulmonary embolism (PE), critical limb ischemia (CLI), carotid artery and stroke interventions, venous interventions, deep vein thrombosis (DVT), and interventional radiology therapies. The focus on most of these therapies is minimally invasive, catheter-based procedures performed in a cath lab.
Displaying 9 - 16 of 889