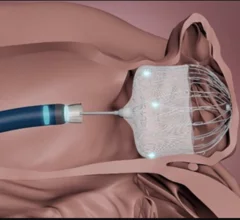
Left Atrial Appendage Closure
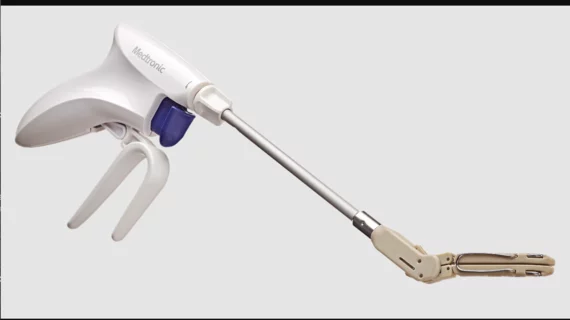
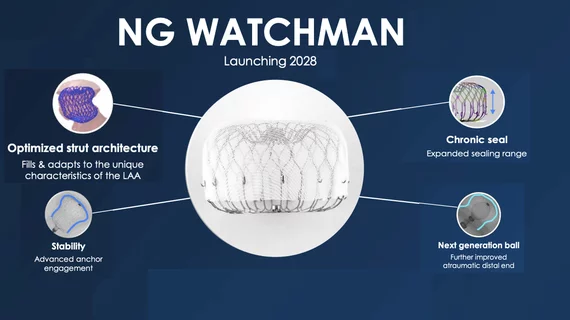
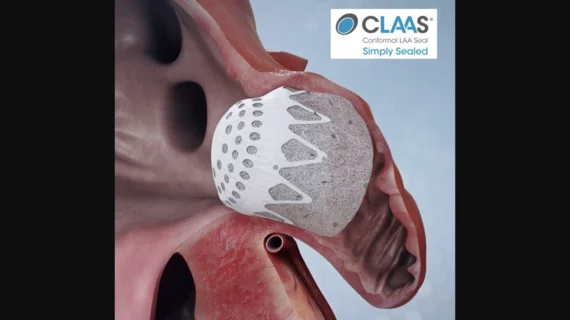
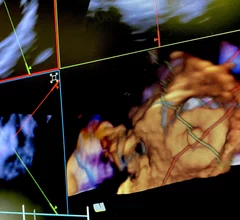
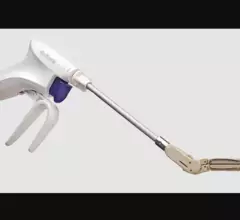
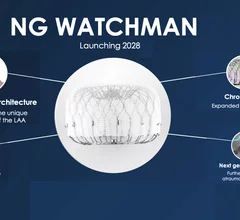
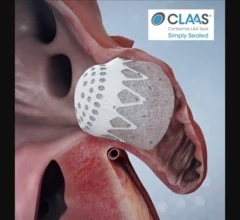
The left atrial appendage (LAA) of the heart is a common location where clots form in atrial fibrillation (AFib) patients that then embolism and cause a stroke. Closing off the LAA either surgically, with a clip or via transcatheter LAA closure device, closes the opening to the LAA to prevent clots from forming or embolizing. The clots are the reason why AFib patients need to be on anticoagulants. LAA occlusion (LAAO) enables patients to stop taking anticoagulation drugs. LAAO has been a rapidly growing segment of structural heart procedures since the approval of the first device, the Watchman, in 2015. Procedures are performed by electrophysiologists, interventional cardiologist or cardiac surgeons.