Intravascular lithotripsy
Intravascular lithotripsy (IVL) helps interventional cardiologists break down heavily calcified plaques in the coronary and peripheral arteries prior to percutaneous coronary intervention. Shockwave Medical, now a part of Johnson & Johnson MedTech, got the ball rolling with its IVL therapies that use shockwave pulses to clear out the calcified plaques. Several other companies have now entered the space, working to develop their own IVL systems that can improve patient outcomes.
Displaying 9 - 16 of 22


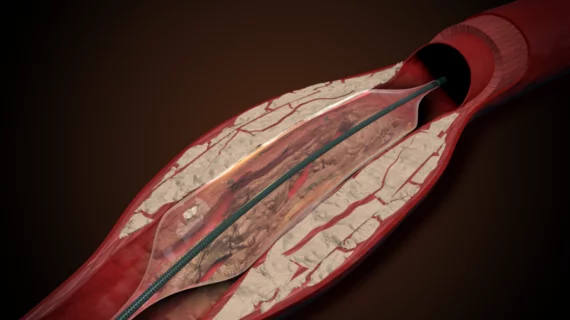
![The use of intravascular lithotripsy (IVL) during percutaneous coronary intervention (PCI) is still safe and effective when patients present with calcified nodules (CNs), according to new long-term data published in EuroIntervention.[1] Researchers compared outcomes from patients with and without CNs, highlighting key similarities in stent expansion and luminal gain.](/sites/default/files/styles/top_stories/public/2024-12/screenshot_2024-12-02_at_11.07.21_am.png.webp?itok=YDi6SyF_)