Electrophysiology
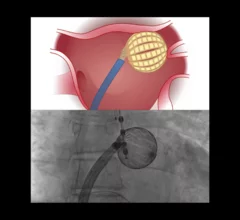
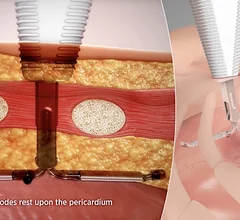
The cardiac subspecialty of electrophysiology (EP) diagnoses and treats arrhythmias. This includes use of pacemakers to treat bradycardia, implantable cardioverter defibrillators (ICD) for tachycardia, heart failure and patients at risk of sudden cardiac arrest, and cardiac ablation treatments to treat heart rhythm disorders.
Displaying 9 - 16 of 434